* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Detoxikace endogenních a exogenních látek
Basal metabolic rate wikipedia , lookup
Peptide synthesis wikipedia , lookup
Butyric acid wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Gaseous signaling molecules wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Microbial metabolism wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Wilson's disease wikipedia , lookup
Proteolysis wikipedia , lookup
Metalloprotein wikipedia , lookup
Glyceroneogenesis wikipedia , lookup
Nitrogen cycle wikipedia , lookup
Biosynthesis wikipedia , lookup
Amino acid synthesis wikipedia , lookup
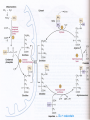
Detoxification of endogenous and exogenous compounds [email protected] A) Detoxification of ammonia Ammonia originates in the catabolism of amino acids that are primarily produced by the degradation of proteins – dietary as well as existing within the cell: digestive enzymes proteins released by digestion of cells sloughed-off the walls of the GIT muscle proteins hemoglobin intracellular proteins (damaged, unnecessary) Nitrogen removal from amino acids transamination oxidative deamination urea cycle Ammonia has to be eliminated: Ammonia is toxic, especially for the CNS, because it reacts with -ketoglutarate, thus making it limiting for the TCA cycle decrease in the ATP level Liver damage or metabolic disorders associated with elevated ammonia can lead to tremor, slurred speech, blurred vision, coma, and death Normal conc. of ammonia in blood: 30-60 µM Transamination Transfer of the amino group of an amino acid to an -keto acid the original AA is converted to the corresponding -keto acid and vice versa: L-alanine pyruvate -ketoglutarate glutamate L-aspartate oxalacetate Transamination is catalyzed by transaminases (aminotransferases) that require participation of pyridoxalphosphate: amino acid pyridoxalphosphate Schiff base Principal transaminations: Alanine transaminase (in the muscle): AA + pyruvate -keto acid + Ala Glutamate transaminase: AA + -ketoglutarate -keto acid + Glu Aspartate transaminase: AA + oxaloacetate -keto acid + Asp Transaminations are usually reversible the actual direction depends on the concentrations of reactants Result: Most of transaminases use -ketoglutarate as an -keto acid, to a lesser extent oxalacetate, thus producing mainly Glu and Asp Glu is either oxidatively deaminated releasing ammonia that – in the liver – enters the urea cycle, or used for syntheses Aspartate enters the urea cycle Oxidative deamination of Glu In mitochondria Glu + NAD(P)+ + H2O → NAD(P)H + H+ + NH4+ + -ketoglutarate Catalyzed by glutamate dehydrogenase that is capable of using both NAD+ and NADP+ Reaction is reversible – either produces Glu, or releases ammonia, depending on the concentrations of reactants Ammonia enters the urea cycle where it is converted to urea Transport of nitrogen – 1) as Gln In the tissues, ammonia is built into Gln by glutamine synthetase: Glu + ATP + NH4+ Gln + H2O + ADP + P Gln is transported to the liver and kidney and deaminated by L-glutaminase: Amide nitrogen, not the -amino nitrogen is removed! Glu can be oxidatively deaminated, ammonia is excreted by the kidneys or converted to urea in the liver Gln in the kidney: A portion of Gln can be taken up by the kidney; another portion of Gln is produced by the kidney itself Ammonia released by the glutaminase reaction in the kidney diffuses into the urine instead of entering the urea cycle These processes participate in the regulation of the acid-base balance and of pH of the urine Transport of nitrogen – 2) as Ala Mainly by the muscle i.a. in the glucose-alanine cycle: Liver Muscle In the fed state, AA released by digestion travel through the hepatic portal vein to the liver and other tissues, where they are used primarily for the synthesis of proteins (in the liver, particularly for the synthesis of plasma proteins): Gln and Ala are the major carriers of nitrogen Upon fasting, some tissues (brain, skeletal muscle, kidney) oxidize Val, Leu, Ile and incorporate nitrogen into Gln, Ala Gln, Ala and other AA carry nitrogen to the liver, kidney, gut, and cells with rapid turnover rate (leukocytes) for biosyntheses (Nt), oxidation, or synthesis of glucose and ketone bodies The unused nitrogen is carried as Ala to the liver to the urea cycle Detoxification of ammonia a) ammonia is built into Glu by glutamate dehydrogenase and Gln by glutamine synthetase: -ketoglutarate + NH4+ + NAD(P)H+H+ Glu + H2O + NAD(P)+ Glu + ATP + NH4+ Gln + H2O + ADP + P Glu, Gln can then be used for syntheses: • Glu – for the synthesis of Gln, Pro, Ala, Asp • Gln – for the synthesis of purines and pyrimidines Transamination Glu + oxalacetate → Asp + 2-oxoglutarate supplies the urea cycle with Asp !!! b) the urea cycle converts ammonia to urea…PRINCIPAL Sources of ammonia for the urea cycle: Oxidative deamination of Glu, accumulated in the liver by the action of transaminases and glutaminase Glutaminase reaction releases NH3 that enters the urea cycle in the liver (in the kidney, it is excreted into the urine) Catabolism of Ser, Thr, and His also releases ammonia: serine dehydratase – NH3 By analogy: Thr to α-ketobutyrate Bacteria in the gut also produce ammonia Urea cycle In the liver in 2 compartments: mitochondrial matrix + cytoplasm In the mitochondrial matrix, oxidative deamination of Glu releases ammonia that is converted to carbamoyl phosphate: NH4+ + HCO3- + 2 ATP 2 ADP + P + carbamoyl phosphate In mitochondria, carbamoyl phosphate reacts with ornithine, yielding citrulline, which is transported to the cytoplasm Ornithine is regenerated by step 5 and transported back to mitochondria Fumarate ← Glu + oxalacetate 3 moles of ATP are required for the formation of 1 mole of urea: 2 for the formation of carbamoyl phosphate 1 for the formation of argininosuccinate Regulation by N-Ac-Glu Carbamoyl phosphate synthetase I (CPSI) is activated by N-acetylglutamate: N-Ac-Glu is synthesized from Glu and AcCoA which can be stimulated by Arg When AA breakdown rises, conc. of Glu and Arg increase the concentration of N-Ac-Glu is also increased activation of CPS I stimulation of the urea cycle Deficiencies of the urea cycle enzymes Lead to elevated Gln and ammonia levels in the circulation 1) N-acetylglutamate synthetase • administration of carbamoyl glutamate (also activates CPSI) 2) CPSI: • administration of benzoate and phenylacetate → hippurate and Phe-Ac-Gln are excreted in the urine: 3) Ornithine transcarbamoylase – the most common deficiency • the same treatment as in the case 2) 4) Argininosuccinate synthetase accumulation of citrulline in the blood and excretion in the urine (citrullinemia) • supplementation with Arg necessary 5) Argininosuccinate lyase: • treatment as in the case 2) + supplementation with Arg 6) Arginase (rare) Arg accumulates and is excreted • administration of benzoate + low protein diet including essential AA (but excluding Arg) or their keto analogs In all cases, the low nitrogen diet is applied Other nitrogenous degradation products excreted in the urine Creatinine – produced from creatine phosphate: Uric acid – degradation product of the purine bases B) Metabolism of xenobiotics Drugs, preservatives, pigments, pesticides … Predominantly in the liver, also in the intestines, kidney, lungs Involves two phases Phase 1 Incorporation of new groups or alteration of groups that are already present in the molecule In the endoplasmic reticulum (ER) Result: increase in the polarity (supports excretion) change in biological activity: • A) decrease in the biological activity (toxicity) • B) activation: some compounds only become biologically active once they have been subjected to phase 1 Potential toxic effects of activated compounds Cytotoxicity – e.g. by covalent binding to proteins Binding to a protein, thus altering its antigenicity antibodies are produced that can damage the cell Carcinogenesis – phase 1 can convert procarcinogens (e.g. benzo[]pyren) to carcinogens. Epoxid hydrolase (in ER) can convert reactive, mutagenic and/or carcinogenic epoxides to less reactive diols: epoxide diol Reactions of phase 1: Hydroxylation Epoxide formation Reduction of carbonyl-, azo-, or nitro- compounds Dehalogenation Hydroxylation Chief reaction of the phase 1 Catalyzed by cytochrome P450s: in humans: ~60 isoenzymes; the most abundant: CYP3A4 monooxygenases: RH + O2 + NADPH + H+ ROH + H2O + NADP+ Electrons from NADPH+H+ are transferred to NADPHcytochrome P450 reductase, then to cytochrome P450 and to oxygen → one oxygen atom is inserted into the substrate They metabolize not only xenobiotics but also endogenous compounds, e.g. some steroids, eicosanoids Isoforms of cytochrome P450 Hemoproteins in the endopl. reticulum, inner mitoch. membrane Most abundant in the liver and small intestine followed by lungs Nomenclature based on the AA sequence identity: CYP3A4 CYP = cytochrom P450 3…family 4…isoform number within the subfamily A…subfamily Some exist in polymorphic forms, some of which exhibit low activity accumulation of the corresponding xenobiotic Some are involved in metabolism of polycyclic aromatic hydrocarbons (PAHs), thus playing a role in carcinogenesis Most isoforms are inducible: E.g. by phenobarbital and other drugs, but also by their own substrates Mechanism: mostly increased transcription Can lead to drug interaction: induction of the particular isoform by the drug 1 (e.g. phenobarbital) can speed up metabolism of the drug 2 (e.g. warfarin) by this isoform it is necessary to increase the dose of the drug 2 Metabolism of ethanol – mainly in the liver Most of acetate enters the blood and, mainly in the skeletal muscle, is activated to acetyl-CoA → TCA cycle The other route (~10-20%): by the cyt P450 isoform CYP2E1: CH3CH2OH + NADPH+H+ + O2 → NADP+ + 2 H2O + CH3CHO Acetaldehyde can enter the blood and damage tissues. CYP2E1 is induced by ethanol and metabolizes also some carcinogenic components of tobacco smoke! Phase 2 – conjugation Products of phase 1 are conjugated with: glucuronate sulphate glutathione Conjugation renders them even more water-soluble and eventually even less active; conjugates are excreted with the bile (conjugates with Mr 300) or urine (Mr 300) Glucuronidation UDP-glucuronic acid is the glucuronate donor: glucuronate Glucuronate can be attached to oxygen (O-glucuronides) or nitrogen (N-glucuronides) groups Excreted as glucuronides are: benzoic acid, meprobamate, phenol, and also endogenous compounds – bilirubin, steroids Bilirubin excretion Bilirubin is the product of heme catabolism: heme M: methyl, V: vinyl, CE: carboxyethyl (propionic) transported to the liver bound to albumin heme → biliverdin → bilirubin transport to the liver (albumin) conjugation with glucuronate bilirubin diglucuronide secreted into the bile bacteria in the small intestine release bilirubin from diglucuronide and convert it to colourless urobilinogens a small fraction is reabsorbed and reexcreted through the liver into the bile a small fraction is excreted into the urine by the kidney most of them are oxidized to pigments and excreted in the faeces (urobilin, stercobilin) Sulfation Some alcohols, arylamines, phenols, but also glycolipids, steroids Sulfate donor: PAPS (3´-phosphoadenosine-5´-phosphosulfate): Conjugation with glutathione Glutathione (GSH) = -glutamylcysteinylglycine: Conjugation with GSH: G–S–H + R G–S–R + H+ (R…electrophilic xenobiotic) Conjugation with GSH prevents binding of distinct xenobiotics to DNA, RNA, or proteins, and subsequent cell damage! Metabolism of glutathione conjugates: Glutamyl and glycinyl are removed from GSH an acetyl group (donated by acetyl-CoA) is added to the amino group of the Cys moiety mercapturic acid (conjugate of acetyl-Cys) is excreted in urine mercapturic acid C) Metallothioneins Small proteins (~ 6,5 kDa), cysteine-rich the –SH groups bind metal ions: Cu2+, Zn2+, Hg2+, Cd2+ In cytosol, mainly of the liver, kidney, and intestine cells Induced by metal ions Functions: binding of metals, regulation of the Zn2+ level, transport of metals (Zn2+)