* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Angiogenesis
Cytokinesis wikipedia , lookup
Cell growth wikipedia , lookup
Cell encapsulation wikipedia , lookup
Signal transduction wikipedia , lookup
Tissue engineering wikipedia , lookup
Organ-on-a-chip wikipedia , lookup
Cell culture wikipedia , lookup
Cellular differentiation wikipedia , lookup
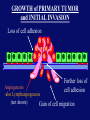
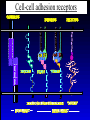
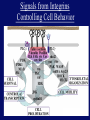
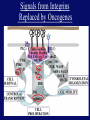
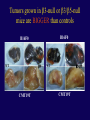
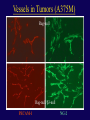
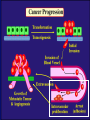
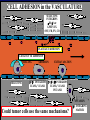
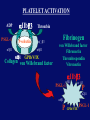
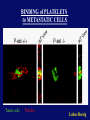
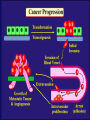
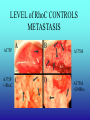
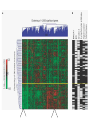
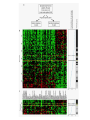
”Cell Adhesion in Tumor Growth, Progression and Angiogenesis" Richard Hynes HHMI/MIT MGH Tumor Microcirculation Course Cambridge, MA June 4, 2003 GROWTH of PRIMARY TUMOR and INITIAL INVASION Loss of cell adhesion Further loss of cell adhesion Angiogenesis -also Lymphangiogenesis (not shown) Gain of cell migration Angiogenesis and Lymphangiogenesis • Essential for growth of primary tumor (and later of metastases) • Involves extensive migration and adhesion of endothelial cells and pericytes • Involves organization of basement membranes Metastatic Spread • Intravasation • Survival in circulation • Arrest at a distant site - selectivity?? • Intravascular Proliferation ? • Extravasation • Survival and proliferation at the new site • Angiogenesis again • All of these involve cell adhesion Cell-Cell and Cell-Matrix Adhesion U CELL-CELL ADHESION e.g., CADHERINS BASEMENT MEMBRANE (MATRIX) CELL-MATRIX ADHESION e.g., INTEGRINS Cell-cell adhesion receptors Cell-matrix adhesion receptors CELL-MATRIX ADHESION INTEGRINS KQAGDV S S S S IIb S S 5 6 RGD FIBRINOGEN FIBRONECTIN LAMININ Connections between extracellular matrix (ECM) and the actin cytoskeleton PLAN VIEW ACTIN ECM SIDE VIEW POINTS of ATTACHMENT The Molecular Linkage Between Actin and ECM via Integrins SIGNALLING EVENTS ACTIN FILAMENTS TALI N PAXILLIN IN S N E T VIN FAK A src A PKC CDK MEMBRANE INTEGRIN MATRIX RGD PROTEOGLYCAN Signals from Integrins Controlling Cell Behavior Functions of Cell Adhesion Receptors • Mediate adhesion to adjacent cells and to ECM • Control cell shape, polarity and migration • Control cell proliferation, survival, gene expression and differentiation How do these functions impact tumor progression? MATRIX/INTEGRINS and GROWTH CONTROL • Integrins regulate cyclin D synthesis • Integrins regulate PIP2 synthesis • Both these effects synergize with stimulation by soluble growth factors • In fact, they are necessary for growth factors to promote growth - cells will not grow with growth factors alone - they need matrix attachment through integrins. • This is “anchorage dependence of growth” MATRIX/INTEGRINS and CELL SURVIVAL • Integrins regulate PI3 kinase and Akt, acting through FAK • This pathway suppresses apoptosis • So extracellular matrix, acting via integrins provides local survival signals • i.e., cells must be attached to the correct matrix in order to survive. • This is “anchorage dependence of survival” ANCHORAGE DEPENDENCE • Most normal cells are dependent on anchorage for survival and proliferation • Tumor cells are not, because oncogenes provide the signals normally provided by integrins and other adhesion receptors • So tumor cells are less dependent on being attached in the correct place Signals from Integrins Replaced by Oncogenes Angiogenesis Necessary for growth and survival of both primary and metastatic tumors v Integrins (v and v) in Angiogenesis • upregulated on (many) angiogenic vessels • Inhibitors - some antibodies (LM609) and RGDbased peptides and peptidomimetics block angiogenesis and induce apoptosis in various model systems • MODEL:- v & vintegrins are proangiogenic and potential targets for antiangiogenesis therapy Predictions from this model • Mice lacking v integrins should show defects in angiogenesis embryonic lethal but lacks a dozen integrins v All three are viable and fertile either as single KOs or as double KOs v and 8 KOs show extensive angiogenesis, although they are not viable • So the simple predictions are not met Conclusions from integrin knockouts • embryos of v-null mice generally show normal vascular development • the selective vascular defects in the brain are of neural/glial origin • the KO mouse has similar defects • in any event, they are not due to absence of vand/or v ( vand/or v are NO ESSENTIAL for normal vascular development What about tumor angiogenesis? • Transplantable tumors Human: • LS180: colon carcinoma • A375SM: melanoma Mouse: • CMT19T: lung carcinoma • B16FO: melanoma • Endogenous tumors • RIPTAg • MMTV-neu Tumors grown in -null or -null mice are BIGGER than controls B16F0 B16F0 -/- WT CMT19T -/- WT CMT19T Tumors grown in -null or -null mice are BIGGER than controls B16F0 p< 0.02 CMT19T p< 0.01 A375SM p< 0.02 Vessels in Tumors (A375M) Rag-null Rag-null/3-null PECAM-1 NG-2 Tumors grown in -null or -null mice have MORE VESSELS than controls B16F0 tumor Normal skin Tumor Growth and Angiogenesis WT KO DKO B16 melanoma + ++ ++ CMT19T lung carcinoma + ++ ++ LS180 adenocarcinoma + ++ ++ A375M melanoma + ++ ++ C57BL/6 Rag2 So:- tumor growth and angiogenesis are NOT dependent on v or v. In fact, these integrins tend to inhibit them. HOW? 51 Integrin and Fibronectin in Angiogenesis • both are upregulated on angiogenic vessels • mice lacking 51 die with vascular defects • mice lacking die with vascular defects • antibodies to either inhibit angiogenesis • peptides blocking their interaction inhibit angiogenesis • that is - genetics and inhibitor studies conform here • Fibronectin and 51 integrin are proangiogenic • They appear good targets for antiangiogenesis A new way of thinking about v integrins in angiogenesis • The original model of their being proangiogenic does not explain all the data • Perhaps they are actually antiangiogenic or negative regulators some or all the time • The negative regulation model does a better, although not a perfect job of explaining the data Possible Negative Regulation by v Integrins Transdominant Inhibition ENDOTHELIAL CELL 11 21 COLLAGENS LAMININS v3 v5 FIBRONECTIN VITRONECTIN FIBRINOGEN OSTEOPONTIN von WILLEBRAND FACTOR THROMBOSPONDIN 51 FIBRONECTIN SELECTIVE INHIBITION Based on data showing cross- inhibition by ligation of different integrins on the same cell. Works best when the inhibitory integrin is at a high level Like v and v ! Agonists orAntagonists? • That often depends on the assay • The same agent can act as an agonist when presented on a substrate and an antagonist when presented in solution • An agent detected as an antagonist in an adhesion assay can be an agonist with respect to signaling Design of anti-v integrin drugs • It is not enough just to screen for antagonists of adhesion • Figure out the (positive and negative) functions of v and v • for their ability to stimulate the negative or inhibit the positive pathways - that is, agonists or antagonists LOSS of ADHESION for HOME BASE LOSS of CELL-CELL ADHESION PROTEINS (CADHERINS) CONTRIBUTES to INVASION of COLON and STOMACH CANCERS GAIN of NEW ADHESION GAIN of NEW CELL ADHESION PROTEINS (INTEGRINS ) CONTRIBUTES to INVASION of MALIGNANT MELANOMA Cadherins and Integrins in Tumor Invasion • Cadherins, particularly E-cadherin, are frequently lost from invasive malignant tumors • Integrins are sometimes gained by invasive tumors • This reflects the switch from sessile adherent epithelial cells to migratory, invasive mesenchymal cells • Often called the Epithelial-Mesenchymal Transition or EMT CADHERINS EPITHELIALMESENCHYMAL TRANSITION HGF/SF Met KERATINS VIMENTIN FIBRONECTIN Common to development and tumor progression RELEASE of -CATENIN from CADHERINS ENHANCES TRANSCRIPTION wnt frz dsh P GSK3 APC DEGRADATION - wnt + wnt LEF-1 TRANSCRIPTION rac cdc42 IQGAP calmodulin How do Circulating Tumor Cells Arrest? Mechanical trapping in small vessels? Emboli with host cells and platelets? Specific arrest via cell adhesion? CELL ADHESION in the VASCULATURE SELECTINS INTEGRINS IIb3 etc GPIb/V/IX vWF, FB, FN, CO PLATELET ADHESION LEUKOCYTE ADHESION ROLLING SELECTINS ADHESION 2/1 INTEGRINS ICAMs, VCAM-1 EXTRAVASATION 1/2 / INTEGRINS ICAMs, VCAM-1 PECAM-1 INVASION Could tumor cells use the same mechanisms? 1 INTEGRINS MATRIX SELECTINS and METASTASIS • Acquisition by human carcinomas of carbohydrate ligands (S-Lex and S-Lea) for selectins is associated with poor prognoses • Selectins are expressed by vascular cells platelets, leukocytes, endothelium • Could tumor cells use selectins in their metastatic spread? S-Lex S-Lex S-Lex S-Lex PLATELETS and METASTASIS • Platelets enhance metastatic spread • HOW? Provision of adhesion molecules • Adherence to tumor cells? • Bridging between tumor cells and endothelium ? • Provision of growth factors/cytokines • Protection against turbulence • Trapping of embolus • Could selectins or integrins play a role? PLATELET ACTIVATION ADP PSGL-1 IIb P-selectin Thrombin GPIb/V/IX Collagen von Willebrand factor Fibrinogen von Willebrand factor Fibronectin Thrombospondin Vitronectin IIb PSGL-1 P P GPIb/V/IX PSGL-1 SELECTINS, LIGANDS, PLATELETS and METASTASIS S-Lex S-Lex S-Lex PLATELETS S-Lex S-Lex S-Lex S-Lex S-Lex S-Lex S-Lex S-Lex S-Lex S-Lex S-Lex S-Lex FIBRINOGEN S-Lex S-Lex ENHANCED ADHESION and TRAPPING of TUMOR CELLS ?? SELECTIN-DEFICIENT MICE Chr 1 All three genes ablated in all combinations P L E Stephen Robinson All strains viable and fertile INTRAVENOUS INJECTION of TUMOR CELLS - SCORE LUNG METASTASES • Mice lacking one, two or all three selectins • C57BL6 background to investigate murine tumors (eg.,MC38 colon adenocarcinoma) • Rag2-/- background to investigate human tumors (eg.LS180 adenocarcinoma) • These cells express ligands for all 3 selectins Daniela Taverna and collaboration with Ajit Varki/Lubor Borsig SELECTIN DEPENDENCE of METASTASIS to LUNGS LS180 COLON CARCINOMA CELLS - Rag2-/- BACKGROUND Alu PCR WT SELECTIN DEPENDENCE of METASTASIS to LUNGS MC38 ADENOCARCINOMA CELLS -C57BL6 BACKGROUND SELECTIN DEPENDENCE of METASTASIS to LUNGS MC38 ADENOCARCINOMA CELLS - C57BL6 BACKGROUND (GFP) SELECTINS and EXPERIMENTAL METASTASIS to LUNGS • P and L selectins both enhance metastasis and their effects are additive • E-selectin has rather little effect • True for injected tumor cells of either human (LS180) or mouse (MC38) origin • Selectin ligands on the tumor cells may be contributing to metastasis SELECTINS on VASCULAR CELLS Platelets Leukocytes L L Activation P P P L L L L L L L L PPP Activation L (Shedding) L L PPP L P L L L L L L Activation P P P P L L L L L P P (Exocytosis) L L P L L L L PP PPPPPPPPPPPP (Exocytosis) Endothelial Cells Activation (Biosynthesis) EEEEEEEEEEEEE SELECTINS, LIGANDS, PLATELETS, LEUKOCYTES and METASTASIS L S-Lex L S-Lex S-Lex P S-Lex S-Lex P S-Lex S-Lex S-Lex S-Lex P PPP L S-Lex S-Lex x S-Lex S-Le L P Activation PPP L S-Lex S-Lex S-Lex S-Lex (Exocytosis) PP PPPPPPPPPPPP L BINDING of PLATELETS to METASTATIC CELLS Tumor cells Platelets Lubor Borsig HOST CELL ENHANCEMENT of METASTASIS • Likely contributors include platelets and leukocytes binding to the tumor cells • Suggests that reagents blocking selectin interactions might be useful in inhibiting metastatic spread • Need to find out which are the key host cells e.g, bone marrow transplantations SUBCUTANEOUS INJECTION of TUMOR CELLS - SCORE GROWTH of PRIMARY TUMOR • Mice lacking specific selectins • Rag2 background to investigate human tumors (eg.LS180 adenocarcinoma) Subcutaneous injection of LS180 cells into selectin-deficient mice Tumor weight WT P -/p< 0.029 33 days WT E -/p< 0.011 WT ELP-/p< 0.0001 Daniela Taverna DEPENDENCE on PRESENCE of L- SELECTIN LS180 cells Rag-2-null background 30 days Lubor Borsig SELECTINS and GROWTH of PRIMARY TUMORS • Deficiencies in P, L and E-selectins all enhance tumor growth and the effects are additive • True for several different tumor cell lines • Suggests some anti-tumor role for leukocytes • Rag-2 -/- mice lack B, T and NK-T cells • Macrophages, NK cells, platelets, endothelium ??? BONE MARROW TRANSPLANTATION FOLLOWED by TEST for TUMOR GROWTH 1. Irradiate Rag-2-null mice WT or Selectin-deficient 2. Reconstitute with Bone marrow WT or Selectin-deficient 3. After recovery Inject with tumor cells and assay Tumor growth Enhanced tumor growth in ELP-null mice is greatly REDUCED by irradiation and reconstitution with Rag2-/- bone marrow Daniela Taverna Rag2BM n=3 n=5 n=7 n=5 CONCLUSIONS from BONE MARROW TRANSPLANTS • Mice with selectin-deficient bone marrows consistently yield larger tumors • Some selectin-dependent BM-derived cells suppress tumor growth • Macrophages and NK cells express L-selectin and PSGL-1 • Endothelium expresses P- and E-selectins • Platelets express P-selectin and PSGL-1 platelets could also recruit other cell types How do metastatic cells arise? Are they all the same? Is there specificity in their arrest? Or is there specificity in their ability to grow/survive in distant sites? Cell Line Cells Injected Tumors in Lungs A375P 5x105 0,0,0,0,5 A375M1 1x105 all 50+ A375M2 2x105 ~50 B16F0 5x104 0,0,0,3,5 B16F1 5x104 50+ to solid B16F2 2.5x104 all 50+ PLAUSIBLE CLUSTERS of ALTERED GENES ~10,000 genes screened 32 are upregulated in metastases F EXTRACELLULAR MATRIX ASSEMBLY fibronectin, collagenI2, collagenIII1, biglycan, fibromodulin F CYTOSKELETAL ORGANIZATION fibronectin, RhoC, thymosin 4 -catenin, -actinin, -centractin, IQGAP-1, calmodulin F ANGIOGENESIS fibronectin t-PA, angiopoietin 1, TGF family THREE "TOP" HITS F FIBRONECTIN Extracellular matrix protein. Known to promote cell proliferation and cell survival Known to promote cell migration Known to promote angiogenesis Upregulated in some other metastatic cells F THYMOSIN 4 Regulator of actin polymerization Other thymosins previously connected to metastasis F RhoC Small GTPase - known to regulate actin cytoskeleton Correlates with invasion and metastasis in human cancers LEVEL of RhoC CONTROLS METASTASIS A375P A375P + RhoC A375M A375M +DNRho Pulmonary Met ast ases Cell Line # of Metastases # of Mice A3 7 5P 0 ,0 ,0 ,0 ,0 ,1 ,5 ,1 0 8 A3 7 5 M all >1 0 0 8 A3 7 5 P-RhoC 5 6 ,7 0 ,>1 0 0 , >1 0 0 4 A3 7 5 M-DNrho 1 3, 24, 29, 32 4 SMALL GTPases in INVASION and METASTASIS INTEGRINS & GROWTH FACTORS (PDGF) INTEGRINS & GROWTH FACTORS (LPA) Tiam-1 cdc42 rac IQGAP-1 cadherin -catenin -catenin rho CYTOSKELETAL ORGANIZATION CELL ADHESION ROCK etc CONTRACTILITY CELL SHAPE & MOTILITY INVASION & METASTASIS Van’t Veer et al, Nature 415:530-536 (2002) Primary breast carcinomas Can identify an expression profile that correlates with incidence of metastases Suggests bulk primary tumor already has properties that predispose to metastasis That is, not (only) rare variant metastatic cells Ramaswamy et al, Nature Genetics 33: 49-54 (2003) Miscellaneous collection of 12 metastases and 64 primary tumors of same histological types - all adenocarcinomas Can identify an expression profile of 128 genes that distinguishes primaries from metastases Some primaries show the “metastasis pattern” Analyzed available data sets and found that the 128 gene set could split primaries into two sets, one of which showed the “metastasis pattern” and had poor prognosis - same result with a 17 gene set Suggests bulk primary tumors already have properties that predispose to metastasis That is, not (only) rare variant metastatic cells 128 gene signature 17 gene signature Clustered by all genes 17 gene signature Kang/Massague et al Cancer Cell (in press). Breast cancer cell line MDA-MB-231 Select variants highly metastatic to bone They “breed true” They have a characteristic expression profile Transfection of 2 or 3 of the overexpressed genes -> increased metastasis Random isolation and screening of clones from parent line identifies clones with the “metastatic signature” These unselected clones ARE metastatic Therefore there ARE preexisting variant cells in the parent population The “metastatic signature” is overlaid on the “poor prognosis signature” of van’t Veer Contrasting Models for Metastatic Progression a. Good prognosis Poor prognosis Metastasis Metastatic variants Metastasis b. Good prognosis c. Good prognosis Poor prognosis Metastasis Hynes, Cell, in press 2003 A More Elaborate Model for Metastatic Progression Good prognosis Stromal response Metastatic variants Poor prognosis Metastasis Hynes, Cell, in press 2003 SS SS SS SS SS SS CELL ADHESION INHIBITORS SS SS SS SS CARBOHYDRATES PEPTIDES ANTIBODIES S S S S