Chapter 7 Section 1 - School District 27J
... use quantum numbers. These represent the energy states of the electron. ...
... use quantum numbers. These represent the energy states of the electron. ...
41 Chapter 4 Atomic Structure 4.1 The Nuclear Atom J. J. Thomson
... This section can be confusing if you don't read it carefully. Beiser estimates the size of a nucleus based on how close a highly energetic alpha particle can get. It may not be clear at first why the distance of closest approach of a particular alpha particle to the nucleus should tell us much about ...
... This section can be confusing if you don't read it carefully. Beiser estimates the size of a nucleus based on how close a highly energetic alpha particle can get. It may not be clear at first why the distance of closest approach of a particular alpha particle to the nucleus should tell us much about ...
Atomic Structure Study Guide
... (2) Atoms of a given element are ___________ in all ways. (3) Atoms of different elements have different physical and chemical __________. (4) Atoms of different elements combine in simple, whole-number ratios to form _________. (5) In chemical reactions, atoms are rearranged, but are never ________ ...
... (2) Atoms of a given element are ___________ in all ways. (3) Atoms of different elements have different physical and chemical __________. (4) Atoms of different elements combine in simple, whole-number ratios to form _________. (5) In chemical reactions, atoms are rearranged, but are never ________ ...
Radiation in the Earth`s Atmosphere Part 1 - IMPRS-gBGC
... Electromagnetic radiation traveling through a medium may be absorbed. The absorption is proportional to the intensity I. It is characterised by the absorption coefficient α. The medium may also emit electromagnetic radiation. This is the source term S. ...
... Electromagnetic radiation traveling through a medium may be absorbed. The absorption is proportional to the intensity I. It is characterised by the absorption coefficient α. The medium may also emit electromagnetic radiation. This is the source term S. ...
nuc_alchemy_talk-fgs-dec07
... Making Gold : Nuclear Alchemy Dr. Paddy Regan Department of Physics University of Surrey Guildford, GU2 7XH [email protected] ...
... Making Gold : Nuclear Alchemy Dr. Paddy Regan Department of Physics University of Surrey Guildford, GU2 7XH [email protected] ...
Nuclear and Modern Physics
... These states are found only at certain energies; we say they are exact, whole. We call these steady states quantum states. ...
... These states are found only at certain energies; we say they are exact, whole. We call these steady states quantum states. ...
PPT - Weizmann Institute of Science
... Analysis: At 10 keV the de Broglie wavelength of 4He is 0.0027 a.u. a 1o grazing angle implies p┴~p║/60 so a perpendicular wavelength of 0.16 a.u.. The angular resolution is ~0.01o so that the coherence wavelength is ~16 a.u., enough to observe diffraction. ...
... Analysis: At 10 keV the de Broglie wavelength of 4He is 0.0027 a.u. a 1o grazing angle implies p┴~p║/60 so a perpendicular wavelength of 0.16 a.u.. The angular resolution is ~0.01o so that the coherence wavelength is ~16 a.u., enough to observe diffraction. ...
topic-2.doc
... o Protons: +1 electrostatic charge o Electrons: -1 electrostatic charge Electrically neutral atoms have an equal number of protons and electrons Atomic number: number of protons in an atom (written 11Na) Atomic weight: equal to number of protons and neutrons ...
... o Protons: +1 electrostatic charge o Electrons: -1 electrostatic charge Electrically neutral atoms have an equal number of protons and electrons Atomic number: number of protons in an atom (written 11Na) Atomic weight: equal to number of protons and neutrons ...
Substance - Department of Chemistry | Oregon State University
... Note the negative energy (-1738 kJ) denotes the process is exothermic (energy was given off by the system.) If a 10,000 gram sample of gold absorbed all of this heat released from the water, what would be the change in temperature of the gold sample? The water gave off energy and the gold took in th ...
... Note the negative energy (-1738 kJ) denotes the process is exothermic (energy was given off by the system.) If a 10,000 gram sample of gold absorbed all of this heat released from the water, what would be the change in temperature of the gold sample? The water gave off energy and the gold took in th ...
4. Energy, Power, and Photons
... If the atoms are excited and then emit light, the atomic beam spreads much more than if the atoms are not excited and do not emit. ...
... If the atoms are excited and then emit light, the atomic beam spreads much more than if the atoms are not excited and do not emit. ...
Smallest sliver of time yet measured sees electrons
... They also fired a near-infrared laser pulse, lasting just four femtoseconds (1 femtosecond is 10-15 seconds). This pulse was able to detect an escaping electron as soon as it was freed from the helium atom. Depending on the electromagnetic field of the laser pulse, the electron either accelerated or ...
... They also fired a near-infrared laser pulse, lasting just four femtoseconds (1 femtosecond is 10-15 seconds). This pulse was able to detect an escaping electron as soon as it was freed from the helium atom. Depending on the electromagnetic field of the laser pulse, the electron either accelerated or ...
First of all, do you know any methods to check
... The change of detection angle will change the surface sensitivity. In many case, it is possible to get quantitative analysis of film thickness from the Auger intensity ratios of substrate and the coated material. ...
... The change of detection angle will change the surface sensitivity. In many case, it is possible to get quantitative analysis of film thickness from the Auger intensity ratios of substrate and the coated material. ...
Bohr Model Notes - Northwest ISD Moodle
... levels (orbitals) outside the nucleus. 2 electrons can fit in the first energy level. 8 electrons can fit in the second energy level. 18 electrons can fit in the third energy level. Valence Electrons – electrons found in the outermost energy levels. Magnesium has 2 valence electrons. Rule of E ...
... levels (orbitals) outside the nucleus. 2 electrons can fit in the first energy level. 8 electrons can fit in the second energy level. 18 electrons can fit in the third energy level. Valence Electrons – electrons found in the outermost energy levels. Magnesium has 2 valence electrons. Rule of E ...
The Atom
... given the mass C you can know both the position and velocity of an object with perfect accuracy if given an accurate value for Planck’s constant ...
... given the mass C you can know both the position and velocity of an object with perfect accuracy if given an accurate value for Planck’s constant ...
What is Matter - watertown.k12.wi.us
... sometimes like a consist of freely moving charged particles, (electrons & ions) It is perhaps the most common phase of matter in the Matter is made up of ...
... sometimes like a consist of freely moving charged particles, (electrons & ions) It is perhaps the most common phase of matter in the Matter is made up of ...
What do we call a substance with more than one kind of atom
... passed straight through; some were reflected straight back to the source. The discovery led Rutherford to make several important conclusions. Using the experimental set-up shown above, what conclusions about atoms were made by Rutherford? a. each atom contains electrons b. each atom contains protons ...
... passed straight through; some were reflected straight back to the source. The discovery led Rutherford to make several important conclusions. Using the experimental set-up shown above, what conclusions about atoms were made by Rutherford? a. each atom contains electrons b. each atom contains protons ...
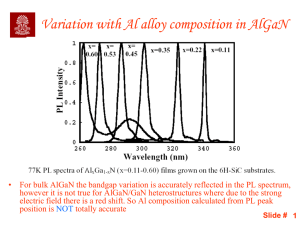
Slide 1
... • For bulk AlGaN the bandgap variation is accurately reflected in the PL spectrum, however it is not true for AlGaN/GaN heterostructures where due to the strong electric field there is a red shift. So Al composition calculated from PL peak position is NOT totally accurate ...
... • For bulk AlGaN the bandgap variation is accurately reflected in the PL spectrum, however it is not true for AlGaN/GaN heterostructures where due to the strong electric field there is a red shift. So Al composition calculated from PL peak position is NOT totally accurate ...
Name: Period
... a. Ionic Solids b. Metallic Solids c. Network Solids 7. How are ionic compounds and molecular compounds different? Ionic Compounds ...
... a. Ionic Solids b. Metallic Solids c. Network Solids 7. How are ionic compounds and molecular compounds different? Ionic Compounds ...
CHE 1401 - Fall 2013 - Chapter 7 Homework 7 (Chapter 7: Periodic
... A) alkali metals have lower densities B) alkali metals have greater electron affinities C) alkali metals have lower ionization energies D) alkali metals have lower melting points E) alkali metals are not more reactive than alkaline earth metals ...
... A) alkali metals have lower densities B) alkali metals have greater electron affinities C) alkali metals have lower ionization energies D) alkali metals have lower melting points E) alkali metals are not more reactive than alkaline earth metals ...
Chapter 6 * Electronic Structure of Atoms
... • Energy (light) is emitted or absorbed in discrete units (quantum) • Each metal has a different energy at when it emits electrons. At lower energy, electrons are not emitted. • Einstein used quanta to explain the photoelectric effect. Energy is proportional to frequency. E = h where h is Planck’s ...
... • Energy (light) is emitted or absorbed in discrete units (quantum) • Each metal has a different energy at when it emits electrons. At lower energy, electrons are not emitted. • Einstein used quanta to explain the photoelectric effect. Energy is proportional to frequency. E = h where h is Planck’s ...
Materialanalytik Praktikum UV-VIS Absorption B507
... Figure 3: A) color wheel. B) Sketch of a single-beam UV/VIS spectrophotometer. C) Sketch of a dualbeam UV/VIS spectrophotometer. D) Image of the UV/VIS/NIR Spectrometer Lambda900 from PerkinElmer. ...
... Figure 3: A) color wheel. B) Sketch of a single-beam UV/VIS spectrophotometer. C) Sketch of a dualbeam UV/VIS spectrophotometer. D) Image of the UV/VIS/NIR Spectrometer Lambda900 from PerkinElmer. ...
9. Time-dependent Perturbation Theory
... conclude that Bab = Bba and A = πω2c~3 Bba. The first of these equations tells us that the rates of absorption and stimulated emission are equal, which we had already found out. The second equation tells us the rate for spontaneous emission in terms of that for stimulated emission, which we already ...
... conclude that Bab = Bba and A = πω2c~3 Bba. The first of these equations tells us that the rates of absorption and stimulated emission are equal, which we had already found out. The second equation tells us the rate for spontaneous emission in terms of that for stimulated emission, which we already ...
CH14 Self Assessment
... of the photoelectric effect predict effect on photoemission when intensity or frequency is changed design investigation and describe analysis for determining work function, threshold frequency, Planck’s constant, stopping voltage, maximum speed, etc. explicitly communicate the relationship between g ...
... of the photoelectric effect predict effect on photoemission when intensity or frequency is changed design investigation and describe analysis for determining work function, threshold frequency, Planck’s constant, stopping voltage, maximum speed, etc. explicitly communicate the relationship between g ...
X-ray fluorescence

X-ray fluorescence (XRF) is the emission of characteristic ""secondary"" (or fluorescent) X-rays from a material that has been excited by bombarding with high-energy X-rays or gamma rays. The phenomenon is widely used for elemental analysis and chemical analysis, particularly in the investigation of metals, glass, ceramics and building materials, and for research in geochemistry, forensic science and archaeology.