* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Chapter 6 * Electronic Structure of Atoms
Bremsstrahlung wikipedia , lookup
Molecular Hamiltonian wikipedia , lookup
Chemical bond wikipedia , lookup
Molecular orbital wikipedia , lookup
Quantum electrodynamics wikipedia , lookup
Ferromagnetism wikipedia , lookup
Tight binding wikipedia , lookup
Double-slit experiment wikipedia , lookup
Hydrogen atom wikipedia , lookup
Auger electron spectroscopy wikipedia , lookup
Particle in a box wikipedia , lookup
Ultrafast laser spectroscopy wikipedia , lookup
X-ray photoelectron spectroscopy wikipedia , lookup
Atomic theory wikipedia , lookup
X-ray fluorescence wikipedia , lookup
Matter wave wikipedia , lookup
Atomic orbital wikipedia , lookup
Wave–particle duality wikipedia , lookup
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
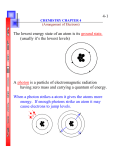
Chapter 6 – Electronic Structure of Atoms Electromagnetic Spectrum (Light) We can use wavelength and frequency to calculate the energy in a photon (particle of light). low frequency high frequency Wavelength (l) is the distance between two crests on a wave. Frequency (f) is the number of waves that pass through a particular point in 1 second (Hz = 1 cycle/s). What is the relationship between energy and frequency? Energy Increases Frequency Increases Energy and frequency are directly related If frequency increases If energy decreases then energy increases then frequency decreases What is the relationship between energy and wavelength? Energy Increases Wavelength Decreases Energy and wavelength are inversely related If wavelength increases then energy decreases If wavelength decreases then energy increases What is the relationship between frequency and wavelength? Frequency Increases Wavelength Decreases Energy and wavelength are inversely related If frequency increases then wavelength decreases If frequency decreases then wavelength increases Why do we care about light in Chemistry?! Electrons release light when they are excited and then calm back down. eLight (Photon – particle of light) Examples of light are gamma rays, x-rays, radio waves, microwaves The particle of light released is called a photon Properties of Light • All electromagnetic radiation travels at the same velocity: Namely the speed of light in a vacuum (c) is 3.00 108 m/s. c = l Ex: A certain AM radio station broadcasts at a frequency of 6.00x102 kHz. What is the wavelength? Properties of Light Light can be defined as both a PARTICLE and a WAVE. Properties of Light • Energy (light) is emitted or absorbed in discrete units (quantum) • Each metal has a different energy at when it emits electrons. At lower energy, electrons are not emitted. • Einstein used quanta to explain the photoelectric effect. Energy is proportional to frequency. E = h where h is Planck’s constant, h = 6.63 10−34 J∙s. Properties of Light Ex: A given light has energy of 2.85x10-19 J. Calculate the frequency and wavelength of that light. The Bohr Model 1. Electrons can have specific (quantized) energy values. 2. Light is emitted as e- moves from one energy to a lower energy level 3. Energy is depicted in the following equation: E = RH ( 1n 2 – i 1 nf2 ) where RH is the Rydberg constant, 2.18 10-18 J, and ni and nf are the initial and final energy levels of the electron. Energy Practice Ex: A photon has absorbed energy when it jumped from n = 2 to n = 4 level. Calculate: a) Energy b) Frequency c) Wavelength of the photon Electron Configurations As chemists, we need to know where an electron is at so we can manipulate it and design awesome stuff Image of iron atoms on copper surface Moving away from the Bohr Model… If negative electrons actually orbited a positive nucleus, they would eventually spiral in and collide, causing an explosion. In reality, the position of an electron is a matter of probability. The space where an electron will probably be found is called an “orbital” Probably here Probably NOT here Quantum Numbers • Four quantum numbers are required to describe the distribution of electrons in atoms. • Each orbital describes a spatial distribution of electron density. Principal Quantum Number (n) • The principal quantum number, n, describes the energy level of the orbital • The values of n = 1,2,3,4… • It gives you the distance of e- from the nucleus Angular Momentum Quantum Number (l) • This quantum number gives the shape of the “volume” of space that the e- occupies • l = 0,1,2,3 … n-1 Magnetic Quantum Number (ml) • The magnetic quantum number describes the orientation of the orbital in space. • ml = −l, … 0, … +l • If l = 1 (p orbital), then ml = -1, 0, or 1 • If l = 2 (d orbital), then ml = -2,-1, 0, 1, or 2 • If l = 3 (f orbital), then ml = -3,-2,-1,0,1, 2, or 3 Relation to Quantum Numbers and Orbitals Magnetic Spin Quantum Number (ms) • Spin quantum number displays the orientation of the electron within the orbital • There can only be 2 electrons in an orbital, where the ms value is either -1/2 or +1/2 +1/2 -1/2 Subshell Shapes These shapes represent where the electrons are probably hanging out The Four Subshells Subshell s Shape Sphere Max # of electrons # of orbitals 2 1 The Four Subshells Subshell p Shape Dumbbell Max # of electrons # of orbitals 6 3 The Four Subshells Subshell d Shape 4-Lobed Max # of # of electrons orbitals 10 5 The Four Subshells Subshell f Shape 6-8 Lobed Max # of electrons 14 # of orbitals 7 Now for a couple rules: Aufbau Principle: add electrons from lowest energy to highest energy (you pretty much already do this when you use the proper filling order) Pauli Exclusion Principle: There is a maximum of two electrons for each line (or orbital) Hund’s Rule: Electrons fill in each orbital first before they start pairing up with an electron Using the periodic table makes it sooooo easy! All you have to do is memorize this: This is what the periodic table would look like if we had more room Or…you can memorize this: What is an electron configuration? It identifies what and where we can expect to find an atom’s electrons The electron configuration for Hydrogen 1 1s Shell (could be 1-7) Subshell (s,p,d,f) Number of e- in subshell s: max of 2 p: max of 6 d: max of 10 f: max of 14 Warm Up 1. What are the 3 orbitals and how many electrons can each orbital hold? 2. What is the next orbital in the series of filling up of the electrons? 1s, 2s, 2p, 3s,3p,__ 3. Given the electron configuration of 1s2 2s2 2p5 What is the element? Writing the e configuration: Steps: 1. Count the number of electrons in the element 2. Use the periodic table to follow the order of filling 3. As you start writing keep count of the electrons used Remember: s [2e-] p [6e-] d [10e-] f [14e-] 4. Check the number of electrons when finished Examples: 1) Carbon [6e-] 2) Sodium [11e-] 1s2 2s2 2p2 1s2 2s2 2p6 3s1 Use the nearest noble gas as a short cut. [He, Ne, Ar, Kr, Xe…] Longhand Configuration Sulfur 16e 2 1s 2 2s 6 2p 2 3s Shorthand Configuration Sulfur 16e 2 4 [Ne] 3s 3p 4 3p Orbital Diagrams • Each box in the diagram represents one orbital. • The arrows represent the electrons, where each arrow indicates the spin