* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Tuesday Aug 19
Ferromagnetism wikipedia , lookup
Particle in a box wikipedia , lookup
Franck–Condon principle wikipedia , lookup
Molecular Hamiltonian wikipedia , lookup
Bremsstrahlung wikipedia , lookup
Chemical bond wikipedia , lookup
Quantum electrodynamics wikipedia , lookup
Molecular orbital wikipedia , lookup
Wave–particle duality wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Mössbauer spectroscopy wikipedia , lookup
Hydrogen atom wikipedia , lookup
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
Electron scattering wikipedia , lookup
X-ray fluorescence wikipedia , lookup
Tight binding wikipedia , lookup
Auger electron spectroscopy wikipedia , lookup
X-ray photoelectron spectroscopy wikipedia , lookup
Electron-beam lithography wikipedia , lookup
Atomic theory wikipedia , lookup
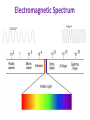
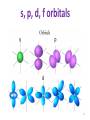
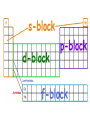
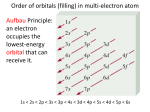
Monday Oct 20 Objective: Write the electronic configuration of any element. Checkpoint: • How many different photons of light can an atom give off if it has 4 energy levels? HW: Emission spectrum lab (due Tuesday) Electromagnetic Spectrum Emission Spectroscopy Lab Pre-lab questions: 1. According to the modern theory of the atom, where may an atom’s electrons be found? 2. How do electrons become “excited”? 3. What form of energy emission accompanies the return of excited electrons to the ground state? 4. Assume that an atom has a total of four possible energy levels and that an electron can jump up or down between any of these energy levels. Draw a model of these energy levels, and use it to predict the maximum number of spectral lines in the emission spectrum. Emission Spectroscopy Lab Data: Sunlight/white light Fluorescent light Emission Spectroscopy Lab Post-lab questions: 1. Compare the spectra produced by sunlight (white light) and fluorescent sources. What are the similarities? What are the differences? 2. Some elements have the same color, but brighter spectral lines than others. How can the difference in the brightness be explained? 3. Prior to its discovery on Earth, the existence of helium was first confirmed in the sun. Explain how this can be possible. Erwin Schrödinger • Quantum Mechanical Model – Modern model – Estimates the probability of finding an electron within a certain volume of space surrounding the nucleus – Where you find the electron 90% of the time 8 Energy Levels • Principal quantum numbers (n) identify the energy levels in an atom – Tells you how far away the electron is from the nucleus – There are 1-7 energy levels, correlates with period numbers – Maximum number of 2n2 electrons per level 9 Atomic Orbitals • Atomic orbitals are more specific regions within each energy level where electrons are found – Highest probability of finding an electron – designated by letters s,p,d,f – Each orbital can hold 2 electrons 10 s, p, d, f orbitals 11 s, p, d, f orbitals Electron Cloud Picture 13 Every atom has energy levels in which electrons can be found. Formula: 2n2 (where n = energy level) Energy Level Total # electrons 1 2 3 4 2 8 18 32 Each orbital can hold 2 electrons Orbital Type # of Orbitals # of total electrons s-orbital 1 2 p-orbital 3 6 d-orbital 5 10 f-orbital 7 14 Writing Electron Configurations Purpose: to summarize where the electrons are (which energy level and orbital) – Electron configuration notation (long form) – Noble gas notation (short form) – Orbital notation (picture form) Write e-configs for: Carbon Magnesium Arsenic Gold