Chapter Summary
... electrons. In contrast to the popular representation of an atom being made up of a positive nucleus orbited by electrons in well-defined circular or elliptical orbits, our modern view is that an electron behaves more like a cloud of negative charge, with the density of the charge cloud at a point co ...
... electrons. In contrast to the popular representation of an atom being made up of a positive nucleus orbited by electrons in well-defined circular or elliptical orbits, our modern view is that an electron behaves more like a cloud of negative charge, with the density of the charge cloud at a point co ...
lesson 6: Heisenberg Uncertainty Principle
... 2. Imagine that you are in a dark room trying to locate a ping pong ball using a metre stick. What happens? Again, your measurement changes the object you are measuring. You can find the ping pong ball’s position at a specific time, but not its velocity. When one measures really small things (on the ...
... 2. Imagine that you are in a dark room trying to locate a ping pong ball using a metre stick. What happens? Again, your measurement changes the object you are measuring. You can find the ping pong ball’s position at a specific time, but not its velocity. When one measures really small things (on the ...
1 - PLK Vicwood KT Chong Sixth Form College
... perpendicular to itself, the brightness does not vary, implying that the light from the lamp is not plane-polarized. (II) A narrow beam of unpolarized light from a slide projector is sent through a tank of water in which scattering particles are obtained by adding one drop of milk. ...
... perpendicular to itself, the brightness does not vary, implying that the light from the lamp is not plane-polarized. (II) A narrow beam of unpolarized light from a slide projector is sent through a tank of water in which scattering particles are obtained by adding one drop of milk. ...
(2)
... relativistic energy-momentum relation. The equation results are called Klein-Garden equation [5]. Another equation based on energy-momentum linear equation is called Dirac. Quantum equations succeeded in describing wide variety of physical phenomena concerning the atomic world [6]. However, there ar ...
... relativistic energy-momentum relation. The equation results are called Klein-Garden equation [5]. Another equation based on energy-momentum linear equation is called Dirac. Quantum equations succeeded in describing wide variety of physical phenomena concerning the atomic world [6]. However, there ar ...
Optical Transitions in Semiconductors
... Curve (a) represents an indirect band gap semiconductor whereas curve (b) represents a direct band gap semiconductor. This can be seen from the slope of the curves. As λ is decreased, which means that the photon energy becomes larger, the absorption of semiconductor (a) increases slowly, i.e. no eff ...
... Curve (a) represents an indirect band gap semiconductor whereas curve (b) represents a direct band gap semiconductor. This can be seen from the slope of the curves. As λ is decreased, which means that the photon energy becomes larger, the absorption of semiconductor (a) increases slowly, i.e. no eff ...
Exam 2 with Solutions - Little Dumb Doctor .Com
... 10. In the Lewis electron dot structure for hydrazine, N2H4, the total number of lone electron pairs around the two nitrogen atoms is c. 2 11. Which compound contains a carbon-oxygen bond with a bond order of 2? a. CO2 12. Using the VSEPR theory, predict the molecular shape of ClF3. b. T-shaped 13. ...
... 10. In the Lewis electron dot structure for hydrazine, N2H4, the total number of lone electron pairs around the two nitrogen atoms is c. 2 11. Which compound contains a carbon-oxygen bond with a bond order of 2? a. CO2 12. Using the VSEPR theory, predict the molecular shape of ClF3. b. T-shaped 13. ...
Diode Pumped Solid State
... able to dominate over absorbtion and spontaneous emission, and secondly there must be an optical cavity. Fortunately both are relatively simple to achieve. The population inversion required for stimulated emission to dominate is realised by increasing the current through the diode. This increases th ...
... able to dominate over absorbtion and spontaneous emission, and secondly there must be an optical cavity. Fortunately both are relatively simple to achieve. The population inversion required for stimulated emission to dominate is realised by increasing the current through the diode. This increases th ...
Thornton/Rex Chp 4 Structure of the Atom
... Thomson’s “plum-pudding” model of the atom had the positive charges spread uniformly throughout a sphere the size of the atom with, the newly discovered “negative” electrons embedded in the uniform background. ...
... Thomson’s “plum-pudding” model of the atom had the positive charges spread uniformly throughout a sphere the size of the atom with, the newly discovered “negative” electrons embedded in the uniform background. ...
Chemistry Questions
... b. 39K 4. An atomic mass unit is defined as exactly a. 1/16 the mass of 12C atom b. 1/12 the mass of 12C atom 5. The total number of electrons in the outer shell (energy level) of a sodium ion is 6. As the number of neutrons in the nucleus of a given atom of an element increases, the atomic number o ...
... b. 39K 4. An atomic mass unit is defined as exactly a. 1/16 the mass of 12C atom b. 1/12 the mass of 12C atom 5. The total number of electrons in the outer shell (energy level) of a sodium ion is 6. As the number of neutrons in the nucleus of a given atom of an element increases, the atomic number o ...
Applications of Coherence by Identity
... We have shown that information can be extracted about an object without detecting the photons that interacted with it. ...
... We have shown that information can be extracted about an object without detecting the photons that interacted with it. ...
In a nuclear reaction
... 2- Elements with atomic # greater then Bi 83 are unstable and are radioactive. 3- Isotopes that are unstable have an unstable ratio of protons and neutrons greater then 1:1 3- TRANSMUTATION- changes to the nucleus of an ...
... 2- Elements with atomic # greater then Bi 83 are unstable and are radioactive. 3- Isotopes that are unstable have an unstable ratio of protons and neutrons greater then 1:1 3- TRANSMUTATION- changes to the nucleus of an ...
Atomic Structure Zumdahl Chemistry Chapter 7
... them. The significance of these results is that matter and energy are not distinct. Energy is really a form of matter, and all matter shows the same types of properties. That is all matter exhibited both particulate and wave properties. Large pieces of matter, exhibit predominantly particulate prope ...
... them. The significance of these results is that matter and energy are not distinct. Energy is really a form of matter, and all matter shows the same types of properties. That is all matter exhibited both particulate and wave properties. Large pieces of matter, exhibit predominantly particulate prope ...
4-1 The lowest energy state of an atom is its ground state. (usually
... A student will be placed one floor higher if and only if there is an available room, which is two grades better. The following chart will take care of all rules for your. ...
... A student will be placed one floor higher if and only if there is an available room, which is two grades better. The following chart will take care of all rules for your. ...
The Quantum Mechanical Model and Electron
... II. Quantum Theory In 1900, __________ and _____________ were seen as different from each other in fundamental ways. Matter was ______________. Energy could come in ______________, with any frequency. Scientists at the time did not understand why the color of an object changed when ______________ it ...
... II. Quantum Theory In 1900, __________ and _____________ were seen as different from each other in fundamental ways. Matter was ______________. Energy could come in ______________, with any frequency. Scientists at the time did not understand why the color of an object changed when ______________ it ...
particles - Prof.Dr.Ümit Demir
... Compton directed an x-ray beam of wavelength 0 toward a block of graphite. He found that the scattered x-rays had a slightly longer wavelength than the incident x-rays, and hence the energies of the scattered rays were lower. The amount of energy reduction depended on the angle at which the x-rays w ...
... Compton directed an x-ray beam of wavelength 0 toward a block of graphite. He found that the scattered x-rays had a slightly longer wavelength than the incident x-rays, and hence the energies of the scattered rays were lower. The amount of energy reduction depended on the angle at which the x-rays w ...
CHEMISTRY CHAPTER 5 OUTLINE NOTES 5.1 – Light and
... o One scientist named Neils Bohr thought of electrons being in “orbit” around the nucleus in much the same manner as Earth is in orbit around the sun. This is sometimes called the planetary atomic model or Bohr’s Model. o So a hydrogen atom should be similar to a solar system consisting of a sun and ...
... o One scientist named Neils Bohr thought of electrons being in “orbit” around the nucleus in much the same manner as Earth is in orbit around the sun. This is sometimes called the planetary atomic model or Bohr’s Model. o So a hydrogen atom should be similar to a solar system consisting of a sun and ...
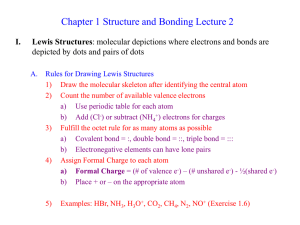
Ch 1 Lecture 2
... a) “Wave Functions” (Y) are solutions to the wave equations b) Y2 = probability of finding e- at each point in space 3) Atomic Orbitals a) e- locations are quantized, not found everywhere but only at certain energies b) 3-D plots of the wave functions indicate where the electron is likely to be foun ...
... a) “Wave Functions” (Y) are solutions to the wave equations b) Y2 = probability of finding e- at each point in space 3) Atomic Orbitals a) e- locations are quantized, not found everywhere but only at certain energies b) 3-D plots of the wave functions indicate where the electron is likely to be foun ...
Quantum Readiness
... The following survey consists of a set of questions about physical and mathematical concepts related to waves and quantum behavior. It is likely that you have been exposed to some of this material to varying degrees in your prior courses. Please respond to the questions to the best of your ability. ...
... The following survey consists of a set of questions about physical and mathematical concepts related to waves and quantum behavior. It is likely that you have been exposed to some of this material to varying degrees in your prior courses. Please respond to the questions to the best of your ability. ...
Modern Physics: Quantum Mechanics
... • The intensity of the beam can be increased by increasing the number of photons/second. • Photons/second = energy/second = power Interaction with matter • Photons interact with matter one at a time. • Energy transferred from photon to matter. • Maximum energy absorbed is photon energy. Phy107 Fall ...
... • The intensity of the beam can be increased by increasing the number of photons/second. • Photons/second = energy/second = power Interaction with matter • Photons interact with matter one at a time. • Energy transferred from photon to matter. • Maximum energy absorbed is photon energy. Phy107 Fall ...
Document
... What actually happened during illumination is that radiation offered enough energy for electrons to overcome binding energy and thus be released. In addition, radiation offered released electrons enough kinetic energy to transfer to the anode surface and overcome repulsion forces with the negative ...
... What actually happened during illumination is that radiation offered enough energy for electrons to overcome binding energy and thus be released. In addition, radiation offered released electrons enough kinetic energy to transfer to the anode surface and overcome repulsion forces with the negative ...
X-ray fluorescence

X-ray fluorescence (XRF) is the emission of characteristic ""secondary"" (or fluorescent) X-rays from a material that has been excited by bombarding with high-energy X-rays or gamma rays. The phenomenon is widely used for elemental analysis and chemical analysis, particularly in the investigation of metals, glass, ceramics and building materials, and for research in geochemistry, forensic science and archaeology.