Midterm Review Date
... 37. What is the total number of oxygen atoms in the formula MgSO 4 • 7 H 2O? [The • represents seven units of H 2O attached to one unit of MgSO 4 .] A) 11 ...
... 37. What is the total number of oxygen atoms in the formula MgSO 4 • 7 H 2O? [The • represents seven units of H 2O attached to one unit of MgSO 4 .] A) 11 ...
Optical Properties of Condensed Matters
... luminesce strongly When electrons are promoted into the excited states of the molecule. The luminescence is Stokes shifted to lower energy compared to absorption, and typically occurs in the middle of the visible spectral region. The emission wavelength can be tuned by small alternation to the chemi ...
... luminesce strongly When electrons are promoted into the excited states of the molecule. The luminescence is Stokes shifted to lower energy compared to absorption, and typically occurs in the middle of the visible spectral region. The emission wavelength can be tuned by small alternation to the chemi ...
Physics 121 Hour Exam #5 Solution This exam consists of a five
... This exam consists of a five problems on five pages. Point values are given with each problem. They add up to 99 points; you will get 1 free point to make a total of 100. In any given problem, points are not necessarily evenly divided between the parts. Two pages are supplied at the end of the bookl ...
... This exam consists of a five problems on five pages. Point values are given with each problem. They add up to 99 points; you will get 1 free point to make a total of 100. In any given problem, points are not necessarily evenly divided between the parts. Two pages are supplied at the end of the bookl ...
NAME PRACTICE: QUANTUM CONFIGURATIONS 1) Each of the
... ___31) N2 molecules absorb ultraviolet light but not visible light. I2 molecules absorb both visible and ultraviolet light. Which of the following statements explains the observations? 1) More energy is required to make N2 molecules vibrate than is required to make I2 molecules vibrate 2) More energ ...
... ___31) N2 molecules absorb ultraviolet light but not visible light. I2 molecules absorb both visible and ultraviolet light. Which of the following statements explains the observations? 1) More energy is required to make N2 molecules vibrate than is required to make I2 molecules vibrate 2) More energ ...
Need for Development of Quantum Mechanics
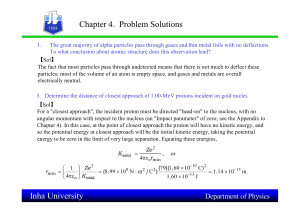
... Calculate de-Broglie wavelength of a proton moving with 1/10th velocity of light. The electron in hydrogen atom may be thought of as confined to a radius of 5x10-11m.Calculate the minimum uncertainty in the momentum of the electron. Also calculate the minimum kinetic energy of the electron. A radar ...
... Calculate de-Broglie wavelength of a proton moving with 1/10th velocity of light. The electron in hydrogen atom may be thought of as confined to a radius of 5x10-11m.Calculate the minimum uncertainty in the momentum of the electron. Also calculate the minimum kinetic energy of the electron. A radar ...
study note 1 06
... What are the different parts of a wave? The part that oscillates between crest and trough and nodes (fixed). What is the restriction placed on the wavelength of standing The only waves that can occur are those for which a half wavelength waves? can be repeated exactly a whole number of times. > Expr ...
... What are the different parts of a wave? The part that oscillates between crest and trough and nodes (fixed). What is the restriction placed on the wavelength of standing The only waves that can occur are those for which a half wavelength waves? can be repeated exactly a whole number of times. > Expr ...
Light in Modern Physics - Physics | Oregon State University
... In the high-frequency case, the more intense the light, the m ore quanta it can transfer, hence the more photoelectrons and the larger the resulting electric current. Of course, to compute this current we don't need to know which particu lar electrons are "lucky." In fact. quantum theory discourage ...
... In the high-frequency case, the more intense the light, the m ore quanta it can transfer, hence the more photoelectrons and the larger the resulting electric current. Of course, to compute this current we don't need to know which particu lar electrons are "lucky." In fact. quantum theory discourage ...
Electromagnetic Spectrum activity
... Wavelength = distance between crests or troughs, symbol λ Amplitude = height of wave Frequency, ν = number of waves that pass a given point per second. The units of waves per second are hertz (Hz) - SI units. Frequency is usually expressed in “waves per second” or 1/s or s-1 e.g. 300 Hz = 300 waves ...
... Wavelength = distance between crests or troughs, symbol λ Amplitude = height of wave Frequency, ν = number of waves that pass a given point per second. The units of waves per second are hertz (Hz) - SI units. Frequency is usually expressed in “waves per second” or 1/s or s-1 e.g. 300 Hz = 300 waves ...
Physical bases of dental material science
... When a molecule is formed, the electrons flow towards the atoms of high electronegativity, the electronegativities of the atoms tend to equalize and acquire the same, uniform value ...
... When a molecule is formed, the electrons flow towards the atoms of high electronegativity, the electronegativities of the atoms tend to equalize and acquire the same, uniform value ...
Lecture-2: Atomic Structure
... What is light? Light exhibits either wave characteristics or particle (photon) characteristics, but never both at the same time. The wave theory of light and the quantum theory of light are both needed to explain the nature of light and therefore complement each other. ...
... What is light? Light exhibits either wave characteristics or particle (photon) characteristics, but never both at the same time. The wave theory of light and the quantum theory of light are both needed to explain the nature of light and therefore complement each other. ...
Section 3: Modern Atomic Theory Atoms Section 3
... electrons are located. Each energy level may contain only a certain number of electrons. The electrons in an atom’s outer energy level are called valence electrons, which determine the chemical properties of an atom. The diagram below shows how many electrons can be found in each of the first four e ...
... electrons are located. Each energy level may contain only a certain number of electrons. The electrons in an atom’s outer energy level are called valence electrons, which determine the chemical properties of an atom. The diagram below shows how many electrons can be found in each of the first four e ...
ppt file - University of Utah Physics
... • Dependence of yield and spectrum on pressure and atmospheric impurities will be measured. • Shower developments equivalent to ~1018 eV will be measured at various depths and compared with codes. • We hope that FLASH will help to shed light on the apparent differences between HiRes and AGASA, and ...
... • Dependence of yield and spectrum on pressure and atmospheric impurities will be measured. • Shower developments equivalent to ~1018 eV will be measured at various depths and compared with codes. • We hope that FLASH will help to shed light on the apparent differences between HiRes and AGASA, and ...
TAP501-0: Spectra and energy levels
... emitted and the energy carried by that photon. Measurements are made of the minimum pd required to just turn an LED on and of the wavelength of light from it. The wavelength may be better taken directly from the manufacturer's specification. Each LED emits photons of one characteristic frequency, sp ...
... emitted and the energy carried by that photon. Measurements are made of the minimum pd required to just turn an LED on and of the wavelength of light from it. The wavelength may be better taken directly from the manufacturer's specification. Each LED emits photons of one characteristic frequency, sp ...
Spectra and energy levels - Teaching Advanced Physics
... emitted and the energy carried by that photon. Measurements are made of the minimum pd required to just turn an LED on and of the wavelength of light from it. The wavelength may be better taken directly from the manufacturer's specification. Each LED emits photons of one characteristic frequency, sp ...
... emitted and the energy carried by that photon. Measurements are made of the minimum pd required to just turn an LED on and of the wavelength of light from it. The wavelength may be better taken directly from the manufacturer's specification. Each LED emits photons of one characteristic frequency, sp ...
5th Grade Energy Study Guide
... 1. ________________ Back and forth motion. (This is what causes sounds to occur). 2. ________________ An area of bunched-up and spread-out particles that move outward in all directions. 3. ________________ A sound wave must have this to travel. (Example- Solid, Liquid, or Gas.) 4. ________________ T ...
... 1. ________________ Back and forth motion. (This is what causes sounds to occur). 2. ________________ An area of bunched-up and spread-out particles that move outward in all directions. 3. ________________ A sound wave must have this to travel. (Example- Solid, Liquid, or Gas.) 4. ________________ T ...
Chapter 5
... Classic physics is what you get when you add up the effects of millions of packages. Quantum mechanics is based on ...
... Classic physics is what you get when you add up the effects of millions of packages. Quantum mechanics is based on ...
1 - M*W
... a) Are the last column (to the right) on the periodic table b) Do not react with other elements c) Are the first column (on the left) on the periodic table d) B & C 34) Halogens, like fluorine, are very reactive because a) They want to gain an electron to complete their outer energy level b) They wa ...
... a) Are the last column (to the right) on the periodic table b) Do not react with other elements c) Are the first column (on the left) on the periodic table d) B & C 34) Halogens, like fluorine, are very reactive because a) They want to gain an electron to complete their outer energy level b) They wa ...
X-ray fluorescence

X-ray fluorescence (XRF) is the emission of characteristic ""secondary"" (or fluorescent) X-rays from a material that has been excited by bombarding with high-energy X-rays or gamma rays. The phenomenon is widely used for elemental analysis and chemical analysis, particularly in the investigation of metals, glass, ceramics and building materials, and for research in geochemistry, forensic science and archaeology.