Waves and the Bohr model
... notice that each element has a unique set of lines and that the colors are distinct wavelengths. This indicates that the electrons in the atoms are changing in energy by discrete amounts (remember we can relate energy and frequency). So we go in the lab and look at lots of spectra. Very complicated. ...
... notice that each element has a unique set of lines and that the colors are distinct wavelengths. This indicates that the electrons in the atoms are changing in energy by discrete amounts (remember we can relate energy and frequency). So we go in the lab and look at lots of spectra. Very complicated. ...
Sensitivity of the FluoroLog® and FluoroMax
... water purified in a Millipore Simplicity® 185. Excitation of the sample was from 250–600 nm, with 50 nm increments. Each emission scan used a step size of 1 nm. Scans were taken under ambient room conditions. ...
... water purified in a Millipore Simplicity® 185. Excitation of the sample was from 250–600 nm, with 50 nm increments. Each emission scan used a step size of 1 nm. Scans were taken under ambient room conditions. ...
ppt
... rainbow of colors without any spectral line • Law 2 – emission line spectrum: a hot, transparent gas produces an emission line spectrum – a series of bright spectral lines against a dark background • Law 3 – absorption line spectrum: a relatively cool, transparent gas in front of a source of a conti ...
... rainbow of colors without any spectral line • Law 2 – emission line spectrum: a hot, transparent gas produces an emission line spectrum – a series of bright spectral lines against a dark background • Law 3 – absorption line spectrum: a relatively cool, transparent gas in front of a source of a conti ...
Introduction to Atomic Spectroscopy
... enough energy to cause electronic excitation, emission takes place in all directions. The emitted radiation from the first excited electronic level, collected at 90o to the incident beam, is called resonance fluorescence. Photons of the same wavelength as the incident beam are emitted in resonance f ...
... enough energy to cause electronic excitation, emission takes place in all directions. The emitted radiation from the first excited electronic level, collected at 90o to the incident beam, is called resonance fluorescence. Photons of the same wavelength as the incident beam are emitted in resonance f ...
Quantum Mechanical Model
... Principal quantum number (n) – indicates the relative size and energy of atomic orbitals ...
... Principal quantum number (n) – indicates the relative size and energy of atomic orbitals ...
ATR Accessories: An Overview
... this accessory but the most common being a top-mounted prism (Figure 2) that will allow the analysis of solids, liquids and pastes. Liquid samples are easy to analyze by pouring a small amount of the sample into a trough plate or casting a film. For routine applications conducted within the industry ...
... this accessory but the most common being a top-mounted prism (Figure 2) that will allow the analysis of solids, liquids and pastes. Liquid samples are easy to analyze by pouring a small amount of the sample into a trough plate or casting a film. For routine applications conducted within the industry ...
Key Concepts for Exam #2
... If the frequency of incident light is above the threshold frequency, then as the intensity of light increases, the kinetic energy of ejected electrons remains constant and the number of electrons increases. In addition, as the frequency of light increases, the kinetic energy of ejected electrons inc ...
... If the frequency of incident light is above the threshold frequency, then as the intensity of light increases, the kinetic energy of ejected electrons remains constant and the number of electrons increases. In addition, as the frequency of light increases, the kinetic energy of ejected electrons inc ...
Exam #2
... mass of the nucleus is concentrated in a very small volume. The electron diffraction experiment demonstrated Heisenberg’s hypothesis that matter and energy are interconvertable. The solution to the Schrodinger wave equation for the hydrogen atom does not provide a detailed description of the electro ...
... mass of the nucleus is concentrated in a very small volume. The electron diffraction experiment demonstrated Heisenberg’s hypothesis that matter and energy are interconvertable. The solution to the Schrodinger wave equation for the hydrogen atom does not provide a detailed description of the electro ...
08_lecture_ppt
... • Bohr theory only modeled the line spectrum of H • Further experiments established waveparticle duality of light and matter – Young’s two slit experiment produced interference patterns for both photons and electrons. ...
... • Bohr theory only modeled the line spectrum of H • Further experiments established waveparticle duality of light and matter – Young’s two slit experiment produced interference patterns for both photons and electrons. ...
08_lecture_ppt - Chemistry at Winthrop University
... • Bohr theory only modeled the line spectrum of H • Further experiments established waveparticle duality of light and matter – Young’s two slit experiment produced interference patterns for both photons and electrons. ...
... • Bohr theory only modeled the line spectrum of H • Further experiments established waveparticle duality of light and matter – Young’s two slit experiment produced interference patterns for both photons and electrons. ...
Looks like ppt is up - Louisiana Tech University
... • notice that B announces his basis AFTER his measurement • if he announced it BEFORE his measurement, then Eve could use the same basis and go undetected. • notice also that EVE can’t store these up and look at them later, because she can’t copy them in the first place (no-cloning) ...
... • notice that B announces his basis AFTER his measurement • if he announced it BEFORE his measurement, then Eve could use the same basis and go undetected. • notice also that EVE can’t store these up and look at them later, because she can’t copy them in the first place (no-cloning) ...
SINGLE-PHOTON ANNIHILATION AND ELECTRON-PAIR
... of the quanta is determined by the obvious requirement i\ .<. l, where i\ = 1/w is the wavelength divided by 2rr, and l the mean distance between particles. It is evident that when i\ < l the concepts of a continuous medium and of a refractive index independent of the position cease to be correct. T ...
... of the quanta is determined by the obvious requirement i\ .<. l, where i\ = 1/w is the wavelength divided by 2rr, and l the mean distance between particles. It is evident that when i\ < l the concepts of a continuous medium and of a refractive index independent of the position cease to be correct. T ...
The Exam 2 Solutions are also available now.
... degenerate with) the state shown in part (a)? (Include the state in (a) in your count.) The energy of an H atom state depends only on the n quantum number. For n = 4, the possibilities are 4s (only 1, m = 0), 4p (3 of them, m = –1, 0, +1), 4d (five of them, m = –2, –1, 0, +1, +2), and 4f (seven of t ...
... degenerate with) the state shown in part (a)? (Include the state in (a) in your count.) The energy of an H atom state depends only on the n quantum number. For n = 4, the possibilities are 4s (only 1, m = 0), 4p (3 of them, m = –1, 0, +1), 4d (five of them, m = –2, –1, 0, +1, +2), and 4f (seven of t ...
Electrons in Atoms
... Begin filling orbitals at the lowest energy level (Aufbau principle) Continue filling, applying Hund’s rule ...
... Begin filling orbitals at the lowest energy level (Aufbau principle) Continue filling, applying Hund’s rule ...
LOC07b Photoelectric Effect Part 2: The Einstein Equation
... Plug in the mercury lamp. It will take a few seconds for it to warm up. The window through which the light enters is covered by filters that only allow certain colors of light to pass. The filters are chosen to coincide with strong emission lines from mercury. The four wavelengths chosen are 390 nm, ...
... Plug in the mercury lamp. It will take a few seconds for it to warm up. The window through which the light enters is covered by filters that only allow certain colors of light to pass. The filters are chosen to coincide with strong emission lines from mercury. The four wavelengths chosen are 390 nm, ...
1/16/2015 Photoelectric Effect Part II The Einstein Equation
... Plug in the mercury lamp. It will take a few seconds for it to warm up. The window through which the light enters is covered by filters that only allow certain colors of light to pass. The filters are chosen to coincide with strong emission lines from mercury. The four wavelengths chosen are 390 nm, ...
... Plug in the mercury lamp. It will take a few seconds for it to warm up. The window through which the light enters is covered by filters that only allow certain colors of light to pass. The filters are chosen to coincide with strong emission lines from mercury. The four wavelengths chosen are 390 nm, ...
Atoms and quantum phenomena
... de Broglie wavelength of an electron • When you accelerate at about 100V then the wavelength is of the order of 10-10m. This is of the same magnitude as an X-ray. We know that X-rays can diffract because of their wave properties and so if this were all true and electrons could exhibit wave behaviou ...
... de Broglie wavelength of an electron • When you accelerate at about 100V then the wavelength is of the order of 10-10m. This is of the same magnitude as an X-ray. We know that X-rays can diffract because of their wave properties and so if this were all true and electrons could exhibit wave behaviou ...
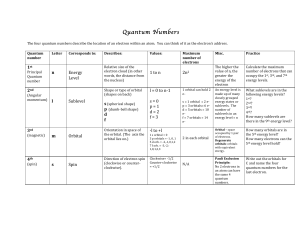
Quantum Numbers Primer The quantum numbers
... n is the principal quantum number (n = 1, 2, 3..., ∞) n designates the principal energy level—it tells us something about the energy (En=-RH/n2 in hydrogen) and the radii (rn=n2a0 in hydrogen). Orbitals with the same value of n are said to be in the same shell. Consider the hydrogen atom. When the e ...
... n is the principal quantum number (n = 1, 2, 3..., ∞) n designates the principal energy level—it tells us something about the energy (En=-RH/n2 in hydrogen) and the radii (rn=n2a0 in hydrogen). Orbitals with the same value of n are said to be in the same shell. Consider the hydrogen atom. When the e ...
Simultaneous Measurements of Hard X Rays and Second
... spectral component centered at photon energies of ø60 keV, while the contribution of lower energy photons to the total x-ray spectrum in the observed spectral window increases rapidly as the energy decreases. In fact, most of the thermal x-ray emission arising from the plasma is expected to peak at ...
... spectral component centered at photon energies of ø60 keV, while the contribution of lower energy photons to the total x-ray spectrum in the observed spectral window increases rapidly as the energy decreases. In fact, most of the thermal x-ray emission arising from the plasma is expected to peak at ...
X-ray fluorescence

X-ray fluorescence (XRF) is the emission of characteristic ""secondary"" (or fluorescent) X-rays from a material that has been excited by bombarding with high-energy X-rays or gamma rays. The phenomenon is widely used for elemental analysis and chemical analysis, particularly in the investigation of metals, glass, ceramics and building materials, and for research in geochemistry, forensic science and archaeology.