Dr. Vikram Panchal Institute Of Chemistry CH-2 Worksheet: -2
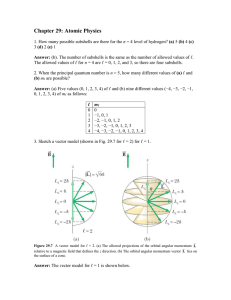
... 8. Given this set of quantum numbers for a multi-electron atom 2, 0, 0,1/2 and 2,0,0,-1/2. What is the next highest allowed set of ‘n’ and ‘l’quantum numbers for this atom in its ground state? 9. The following quantum numbers are responsible for how many orbitals? Given: n=3, l=2, m=2 10. With incre ...
... 8. Given this set of quantum numbers for a multi-electron atom 2, 0, 0,1/2 and 2,0,0,-1/2. What is the next highest allowed set of ‘n’ and ‘l’quantum numbers for this atom in its ground state? 9. The following quantum numbers are responsible for how many orbitals? Given: n=3, l=2, m=2 10. With incre ...
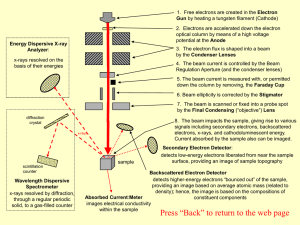
Primary electrons make random elastic and inelastic collision either
... will suffer a “quantum jump” to a low energy state, which will make emission of X-ray photon, and it would be all possible energy up to E0… Secondary electron, (<50 eV, normally around 2-6 eV, larger than sample’s work function) excitations result to loose bound valence electrons, which are promoted ...
... will suffer a “quantum jump” to a low energy state, which will make emission of X-ray photon, and it would be all possible energy up to E0… Secondary electron, (<50 eV, normally around 2-6 eV, larger than sample’s work function) excitations result to loose bound valence electrons, which are promoted ...
ionization chamber
... The different regions of operation of gas filled detectors. The observed amplitude is plotted for events depositing 2 different amounts of energy within the gas ...
... The different regions of operation of gas filled detectors. The observed amplitude is plotted for events depositing 2 different amounts of energy within the gas ...
Modern Physics Lesson 3
... The photoelectric effect showed that a photon, or quantum of light, behaves like a particle, since it has kinetic energy (even though it has no mass). This was a VERY BIG DEAL since light had always been thought of as a wave before. Einstein proposed that photons should also have momentum, and then ...
... The photoelectric effect showed that a photon, or quantum of light, behaves like a particle, since it has kinetic energy (even though it has no mass). This was a VERY BIG DEAL since light had always been thought of as a wave before. Einstein proposed that photons should also have momentum, and then ...
264-lecture-2015-10
... Quantum Mechanics Mnemonic for p “Now I need a drink, alcoholic of course, after the heavy lectures involving quantum mechanics.” ...
... Quantum Mechanics Mnemonic for p “Now I need a drink, alcoholic of course, after the heavy lectures involving quantum mechanics.” ...
List the colors of visible light from low frequency to high frequency
... 1. List the colors of visible light from low frequency to high frequency. 2. Which EMR is the most harmful to living organisms? 3. A. A photon of energy having a wavelength of 1094 nm is released. What energy level was the electron before emitting the photon? B.What energy level is it on after emitt ...
... 1. List the colors of visible light from low frequency to high frequency. 2. Which EMR is the most harmful to living organisms? 3. A. A photon of energy having a wavelength of 1094 nm is released. What energy level was the electron before emitting the photon? B.What energy level is it on after emitt ...
Ch 6 Outline
... Electronic Structure Electromagnetic radiation Speed of light Wavelength Frequency Amplitude Sample exercise 6.1a) and b) ...
... Electronic Structure Electromagnetic radiation Speed of light Wavelength Frequency Amplitude Sample exercise 6.1a) and b) ...
No Slide Title
... x-rays resolved by diffraction, through a regular periodic solid, to a gas-filled counter ...
... x-rays resolved by diffraction, through a regular periodic solid, to a gas-filled counter ...
X-ray fluorescence

X-ray fluorescence (XRF) is the emission of characteristic ""secondary"" (or fluorescent) X-rays from a material that has been excited by bombarding with high-energy X-rays or gamma rays. The phenomenon is widely used for elemental analysis and chemical analysis, particularly in the investigation of metals, glass, ceramics and building materials, and for research in geochemistry, forensic science and archaeology.