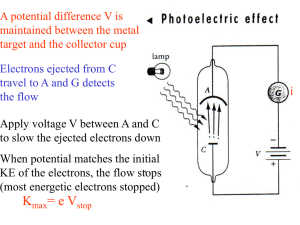
Work sheet –chapter 2 CLASS - XI CHEMISTRY (Structure of Atom
... 3. What is threshold frequency? 4. Name the scientist who demonstrated photoelectric effect experiment. 5. What did Einstein explain about photoelectric effect? 6. What is the relation between kinetic energy and frequency of the photoelectrons? 7. Calculate energy of 2mole of photons of radiation wh ...
... 3. What is threshold frequency? 4. Name the scientist who demonstrated photoelectric effect experiment. 5. What did Einstein explain about photoelectric effect? 6. What is the relation between kinetic energy and frequency of the photoelectrons? 7. Calculate energy of 2mole of photons of radiation wh ...
Document
... - Current which is necessary to restore the charge can be detected - The more radiation that strikes, the less charge remains - Less sensitive than photomultipliers several placed on placed on single crystal ...
... - Current which is necessary to restore the charge can be detected - The more radiation that strikes, the less charge remains - Less sensitive than photomultipliers several placed on placed on single crystal ...
Atomic Structure
... photoelectric effect (1905) was that light is quantized in packets of set energy called photons. (He won the Nobel Prize for this.) • This meant that light had characteristics of particles! ...
... photoelectric effect (1905) was that light is quantized in packets of set energy called photons. (He won the Nobel Prize for this.) • This meant that light had characteristics of particles! ...
Introduction to spectroscopy
... Spectroscopy: Using a probe (radiation, ions or electrons) and sorting its content into energy bins to identify the materials response in each region of the spectrum Recall that any material system made up of atoms, molecules and electrons responds to external stimuli such as light or particles over ...
... Spectroscopy: Using a probe (radiation, ions or electrons) and sorting its content into energy bins to identify the materials response in each region of the spectrum Recall that any material system made up of atoms, molecules and electrons responds to external stimuli such as light or particles over ...
Light problems
... 8.____ A quantum of energy is the a. frequency of electromagnetic energy given off by an atom. b. wavelength of electromagnetic energy gained by an atom. c. minimum quantity of energy that can be lost or gained by an atom. d. continuous spectrum of energy given off by an atom. 9.____ A form of energ ...
... 8.____ A quantum of energy is the a. frequency of electromagnetic energy given off by an atom. b. wavelength of electromagnetic energy gained by an atom. c. minimum quantity of energy that can be lost or gained by an atom. d. continuous spectrum of energy given off by an atom. 9.____ A form of energ ...
Development of the Model of the Atom
... de Broglie wondered that if light has a waveparticle duality, then maybe electrons may have the same nature. Scientists knew any wave confined to a space can have only certain frequencies. De Broglie suggested that electrons be considered waves confined to the space around an atomic nucleus. Exper ...
... de Broglie wondered that if light has a waveparticle duality, then maybe electrons may have the same nature. Scientists knew any wave confined to a space can have only certain frequencies. De Broglie suggested that electrons be considered waves confined to the space around an atomic nucleus. Exper ...
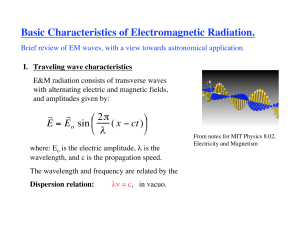
Basic Characteristics of Electromagnetic Radiation.
... Monochromatic flux - is easier to define! Integrate intensity in solid angle over a hemisphere. Thus, flux is the energy in frequency range (ν, ν+Δν) flowing through a unit area per unit time, and into ‘any’ direction (of the hemisphere). ...
... Monochromatic flux - is easier to define! Integrate intensity in solid angle over a hemisphere. Thus, flux is the energy in frequency range (ν, ν+Δν) flowing through a unit area per unit time, and into ‘any’ direction (of the hemisphere). ...
Fourier Transform IR Spectroscopy
... Advantages and Disadvantages • FT – IR can take wavelength readings across the whole IR region simultaneously and smoothly, making this a very rapid technique. • The technique is non-invasive and non-destructive. Its resolution of .125 cm-1 is not spectacular in comparison to other vibrational tech ...
... Advantages and Disadvantages • FT – IR can take wavelength readings across the whole IR region simultaneously and smoothly, making this a very rapid technique. • The technique is non-invasive and non-destructive. Its resolution of .125 cm-1 is not spectacular in comparison to other vibrational tech ...
Modern Physics Review
... the emission of photoelectrons. If the intensity of this radiation is increased, what will happen to the number of the emissions of ...
... the emission of photoelectrons. If the intensity of this radiation is increased, what will happen to the number of the emissions of ...
L 35 Modern Physics [1]
... energy state to a low energy state it emits a photon emission spectrum An electron in a low energy state can absorb a photon and move up to a high energy state absorption spectrum ...
... energy state to a low energy state it emits a photon emission spectrum An electron in a low energy state can absorb a photon and move up to a high energy state absorption spectrum ...
Compton Effect and Spectral Lines
... 1) A photon of initial energy 5.8 103 eV is deflected by 130 in a collision with a free electron, which is initially at rest. What is the wavelength of the scattered photon? What energy (in eV) does the electron acquire in the collision? What is the velocity of the recoil electron? 2) An electron ...
... 1) A photon of initial energy 5.8 103 eV is deflected by 130 in a collision with a free electron, which is initially at rest. What is the wavelength of the scattered photon? What energy (in eV) does the electron acquire in the collision? What is the velocity of the recoil electron? 2) An electron ...
science 1 small-group tutorial scheme
... The hydrogen emission spectrum experiment gives rise to a line spectrum. What does this tell us about the electromagnetic radiation being given out by the hydrogen atom? How was this result explained in terms of the simple Quantum Mechanical (Bohr) model of atomic structure? ...
... The hydrogen emission spectrum experiment gives rise to a line spectrum. What does this tell us about the electromagnetic radiation being given out by the hydrogen atom? How was this result explained in terms of the simple Quantum Mechanical (Bohr) model of atomic structure? ...
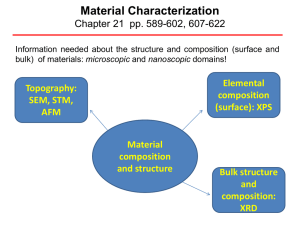
Material Characterization
... X-ray Photoelectron Spectroscopy (XPS) XPS is a surface chemical analysis technique that can be used to analyze the surface chemistry of a material in its "as received" state, or after some treatment XPS detects all elements with an atomic number (Z) of 3 (lithium) and above. It cannot detect hyd ...
... X-ray Photoelectron Spectroscopy (XPS) XPS is a surface chemical analysis technique that can be used to analyze the surface chemistry of a material in its "as received" state, or after some treatment XPS detects all elements with an atomic number (Z) of 3 (lithium) and above. It cannot detect hyd ...
BELLERT_OSU12
... Low Energy Kinetic Measurements of Cluster Ion Decomposition Reactions: Models for Catalysis by Darrin Bellert Baylor University ...
... Low Energy Kinetic Measurements of Cluster Ion Decomposition Reactions: Models for Catalysis by Darrin Bellert Baylor University ...
The Vibrating String
... white light is viewed through a diffraction grating, a continuous spectrum will be produced. ► It looks like this ...
... white light is viewed through a diffraction grating, a continuous spectrum will be produced. ► It looks like this ...
03-02BohrAtom
... What is the energy of the n = 5 orbital? What is the energy of the n = 3 orbital? What is the wavelength of the photon released from a 5 to 3 transition? (Hydrogen atom) ...
... What is the energy of the n = 5 orbital? What is the energy of the n = 3 orbital? What is the wavelength of the photon released from a 5 to 3 transition? (Hydrogen atom) ...
AP Chemistry Chapter 6 Outline for Concepts to Know 6.1 Wave
... 6.1 Wave Nature of Light Basic anatomy and vocabulary of a wave (wavelength, frequency, amplitude) Relationship between speed, wavelength and frequency c= 3.00x108 m/s order of categories of electromagnetic radiation order and approximate range of visible radiation 4 – 8(x10-7) m 6.2 Quant ...
... 6.1 Wave Nature of Light Basic anatomy and vocabulary of a wave (wavelength, frequency, amplitude) Relationship between speed, wavelength and frequency c= 3.00x108 m/s order of categories of electromagnetic radiation order and approximate range of visible radiation 4 – 8(x10-7) m 6.2 Quant ...
South Pasadena • AP Chemistry Name
... Calculate the frequency of the line in the hydrogen spectrum corresponding to the electron transition from n=9 to n=8. Whereabouts in the electromagnetic spectrum does this line occur? ...
... Calculate the frequency of the line in the hydrogen spectrum corresponding to the electron transition from n=9 to n=8. Whereabouts in the electromagnetic spectrum does this line occur? ...
Topics for Final
... Amplitude, wavelength, frequency Electromagnetic radiation, atomic emission spectrum Planck’s Constant = h (E= hv) Photons, photoelectric effect, ground state ...
... Amplitude, wavelength, frequency Electromagnetic radiation, atomic emission spectrum Planck’s Constant = h (E= hv) Photons, photoelectric effect, ground state ...
X-ray fluorescence

X-ray fluorescence (XRF) is the emission of characteristic ""secondary"" (or fluorescent) X-rays from a material that has been excited by bombarding with high-energy X-rays or gamma rays. The phenomenon is widely used for elemental analysis and chemical analysis, particularly in the investigation of metals, glass, ceramics and building materials, and for research in geochemistry, forensic science and archaeology.









![L 35 Modern Physics [1]](http://s1.studyres.com/store/data/001036078_1-1a4f17b9367db590f7dcb987ef21bbe6-300x300.png)