Wave Particle Duality Power Point NOTES
... Electronic structure (electron configurations) - base for many chemistry concepts. ex: molecular geometry & chemical reactivity. ...
... Electronic structure (electron configurations) - base for many chemistry concepts. ex: molecular geometry & chemical reactivity. ...
Modern Physics
... If the energy of a photon is less than the work function f, the photon cannot give enough energy to the electron to leave the surface Kmax does not depend on light intensity, because doubling the number of photons would only double the number of electrons and not double their energy Kmax increases w ...
... If the energy of a photon is less than the work function f, the photon cannot give enough energy to the electron to leave the surface Kmax does not depend on light intensity, because doubling the number of photons would only double the number of electrons and not double their energy Kmax increases w ...
Chapter 12 Worksheet
... a. the momentum of a particle cannot be measured precisely b. neither the position nor the momentum can be measured precisely c. the position and the momentum of a particle can be measured precisely, but not at the same time d. the positon of a particle cannot be measured precisely 4. From the follo ...
... a. the momentum of a particle cannot be measured precisely b. neither the position nor the momentum can be measured precisely c. the position and the momentum of a particle can be measured precisely, but not at the same time d. the positon of a particle cannot be measured precisely 4. From the follo ...
lecture notes, pages 4-5
... Clinton Davisson and Lester Germer (1925) diffracted electrons from a Ni crystal and observed the resulting interference patterns, thus verifying wave behavior of e-‘s. G.P. Thomson had a similar discovery. He showed that electrons that passed through a very thin gold foil produced a diffraction pat ...
... Clinton Davisson and Lester Germer (1925) diffracted electrons from a Ni crystal and observed the resulting interference patterns, thus verifying wave behavior of e-‘s. G.P. Thomson had a similar discovery. He showed that electrons that passed through a very thin gold foil produced a diffraction pat ...
CHAPTER 4 TEST REVIEW GUIDE
... 13. Identify the highest occupied energy level and the number of electrons found there when given an electron configuration. ...
... 13. Identify the highest occupied energy level and the number of electrons found there when given an electron configuration. ...
Final Exam Solutions - University of California San Diego
... An x-ray photon of wavelength 0.02480nm strikes a free stationary electron. The photon scatters off at 90o with respect to the direction of incidence. Determine (a) the momentum of the incident photon (b) the momentum of the scattered photon (c) the kinetic energy of the scattered electron, and (d) ...
... An x-ray photon of wavelength 0.02480nm strikes a free stationary electron. The photon scatters off at 90o with respect to the direction of incidence. Determine (a) the momentum of the incident photon (b) the momentum of the scattered photon (c) the kinetic energy of the scattered electron, and (d) ...
Chapter 22 Instruments for Measuring Apsorption: Is It a Photometer
... Generally, these lamps are operated at a temperature of around 2900 K, which produces useful radiation from about 350 to 2200 nm. Tungsten/halogen lamps, also called quartz/halogen lamps, contain a small amount of iodine within the quartz envelope that houses the filament. Quartz allows the filament ...
... Generally, these lamps are operated at a temperature of around 2900 K, which produces useful radiation from about 350 to 2200 nm. Tungsten/halogen lamps, also called quartz/halogen lamps, contain a small amount of iodine within the quartz envelope that houses the filament. Quartz allows the filament ...
Dr. Harris Chemistry 105 Practice Exam 1 Isotope Atomic Number
... 15. A photon with some energy Ep strikes a metal surface. An electron is ejected with a velocity of 5.00 Mm/s. The threshold frequency of the metal is 6.30 x 1013 s-1. What is the wavelength of the photon, in nm? Significant figures count. 17 nm ...
... 15. A photon with some energy Ep strikes a metal surface. An electron is ejected with a velocity of 5.00 Mm/s. The threshold frequency of the metal is 6.30 x 1013 s-1. What is the wavelength of the photon, in nm? Significant figures count. 17 nm ...
ELECTRONIC STRUCTURE OF ATOMS
... proportional to the frequency of the light. Thus radiant energy was quantized. This explains the difference in effect between xrays(high frequency, high energy) vs radio waves ( low frequency, low energy) Radiation composed of one wave length is called monochromatic. When white light is separate ...
... proportional to the frequency of the light. Thus radiant energy was quantized. This explains the difference in effect between xrays(high frequency, high energy) vs radio waves ( low frequency, low energy) Radiation composed of one wave length is called monochromatic. When white light is separate ...
Name - Red Hook Central Schools
... the work function, φ. The kinetic energy of an ejected photon is the difference between the energy of the incident radiation and the work function, usually given in eV. The “eV” or electron-volt is an energy unit that results when one electron passes through a potential of one volt (Ue = qv). This i ...
... the work function, φ. The kinetic energy of an ejected photon is the difference between the energy of the incident radiation and the work function, usually given in eV. The “eV” or electron-volt is an energy unit that results when one electron passes through a potential of one volt (Ue = qv). This i ...
Quantum Mechanics and the Bohr Model - slater science
... • Draw another horizontal line and two waves with the same wavelength but different amplitudes. ...
... • Draw another horizontal line and two waves with the same wavelength but different amplitudes. ...
Quantum Statistics Applications
... • “normalization” varies with T. Fermi-Dirac easier to generalize • T=0 all lower states fill up to Fermi Energy ...
... • “normalization” varies with T. Fermi-Dirac easier to generalize • T=0 all lower states fill up to Fermi Energy ...
Figure 7.18 The 3d orbitals
... If n > 2 Ry = Rydberg constant = 1.096776 x 107/m If n = 3 get red, n = 4 get green, n = 5 get blue, n =6 get violet Figure 7.8 Three series of spectral lines of atomic hydrogen. Balmer is in the visible region and the other series, which have names also, are in uv or ir area of E-M radiation. The ...
... If n > 2 Ry = Rydberg constant = 1.096776 x 107/m If n = 3 get red, n = 4 get green, n = 5 get blue, n =6 get violet Figure 7.8 Three series of spectral lines of atomic hydrogen. Balmer is in the visible region and the other series, which have names also, are in uv or ir area of E-M radiation. The ...
Introduction to Spectrochemical Methods
... 1. Explain the properties of electromagnetic radiation. 2. Explain the interaction of radiation and matter. 3. Explain radiation absorption and the processes involved. 4. Demonstrate of the limits of Beer’s law. 5. Explain the emission of electromagnetic radiation. 6. Describe the instrument used in ...
... 1. Explain the properties of electromagnetic radiation. 2. Explain the interaction of radiation and matter. 3. Explain radiation absorption and the processes involved. 4. Demonstrate of the limits of Beer’s law. 5. Explain the emission of electromagnetic radiation. 6. Describe the instrument used in ...
visible Ultra violet Infra red Longer line ? Energy? Wavelength
... p 15g × 1kg × 160 km × 10 3 m × 1h 1000g h km 3600s ...
... p 15g × 1kg × 160 km × 10 3 m × 1h 1000g h km 3600s ...
Lec-22_Strachan
... Electrons collected at C and passing through the ammeter create a current in the circuit C is maintained at a positive potential by the power supply No electrons are emitted if the incident light frequency is below some cutoff frequency that is characteristic of the material being illuminated ...
... Electrons collected at C and passing through the ammeter create a current in the circuit C is maintained at a positive potential by the power supply No electrons are emitted if the incident light frequency is below some cutoff frequency that is characteristic of the material being illuminated ...
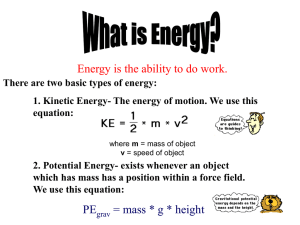
Energy
... Gamma Radiation, g • Released by atoms which have undergone a nuclear reaction. • Results when excited nuclei return to ground state. • High energy! E = hf! ...
... Gamma Radiation, g • Released by atoms which have undergone a nuclear reaction. • Results when excited nuclei return to ground state. • High energy! E = hf! ...
Light
... The human eye can only see the blend of colors. White light is really composed all the above wavelengths. ...
... The human eye can only see the blend of colors. White light is really composed all the above wavelengths. ...
Spectroscopy
... The principle quantum number is n = 1, 2, 3, 4, 5, . . . En is the energy of the nth energy level. The constant R is called the Rydberg constant. Planck’s constant is h; the speed of light is c. In the Bohr Model, the Rydberg constant is predicted to be R 1.0975 x10 7 m 1 . We shall determine R e ...
... The principle quantum number is n = 1, 2, 3, 4, 5, . . . En is the energy of the nth energy level. The constant R is called the Rydberg constant. Planck’s constant is h; the speed of light is c. In the Bohr Model, the Rydberg constant is predicted to be R 1.0975 x10 7 m 1 . We shall determine R e ...
Modern Physics Important Concepts for AP Test
... Electrons can absorb equal to the energy separation, ΔE, to move from the ground state to an excited state (higher energy level). When electron jumps back to a lower level it emits a photon of energy, ΔE. The wavelength is found using ΔE = hf. (Another commonly asked problem on AP test.) o Quant ...
... Electrons can absorb equal to the energy separation, ΔE, to move from the ground state to an excited state (higher energy level). When electron jumps back to a lower level it emits a photon of energy, ΔE. The wavelength is found using ΔE = hf. (Another commonly asked problem on AP test.) o Quant ...
X-ray fluorescence

X-ray fluorescence (XRF) is the emission of characteristic ""secondary"" (or fluorescent) X-rays from a material that has been excited by bombarding with high-energy X-rays or gamma rays. The phenomenon is widely used for elemental analysis and chemical analysis, particularly in the investigation of metals, glass, ceramics and building materials, and for research in geochemistry, forensic science and archaeology.








![L 35 Modern Physics [1] - University of Iowa Physics](http://s1.studyres.com/store/data/001147028_1-f00aa7577568b42bc32948cbade9023a-300x300.png)