
Dr. Harris Chemistry 105 Practice Exam 1 Isotope Atomic Number
... 14. A laser emits 200mJ of energy per hour. Given that the wavelength of the photons in the beam is 300 nm, and assuming that the emission rate is constant, how many photons are emitted per minute? ...
... 14. A laser emits 200mJ of energy per hour. Given that the wavelength of the photons in the beam is 300 nm, and assuming that the emission rate is constant, how many photons are emitted per minute? ...
L 35 Modern Physics [1]
... in allowing us to understand the behavior of big objects such as the motions of the planets, failed when pushed to explain atomic size phenomena. • The discovery of the laws of atomic physics led to every important 20th century discovery that have transformed our lives, the electronic revolution. ...
... in allowing us to understand the behavior of big objects such as the motions of the planets, failed when pushed to explain atomic size phenomena. • The discovery of the laws of atomic physics led to every important 20th century discovery that have transformed our lives, the electronic revolution. ...
Chp.23 Outline - Redlands High School
... frequency will not cause photoelectrons to be released no matter how bright its intensity. What will happen if the intensity of a light above the threshold is increased? What happens if the frequency of the light is increased? Define work function. Write the equation relating the kinetic energy of t ...
... frequency will not cause photoelectrons to be released no matter how bright its intensity. What will happen if the intensity of a light above the threshold is increased? What happens if the frequency of the light is increased? Define work function. Write the equation relating the kinetic energy of t ...
The Particulate Nature of Light
... - e.g., the deflection of a stream of electrons is just like the path of a projectile. Electrons can also exhibit wave properties - e.g., they can demonstrate diffraction. De Broglie proposed that an electron of mass m moving with speed v would have a wavelength given by: = h/p This equation gives ...
... - e.g., the deflection of a stream of electrons is just like the path of a projectile. Electrons can also exhibit wave properties - e.g., they can demonstrate diffraction. De Broglie proposed that an electron of mass m moving with speed v would have a wavelength given by: = h/p This equation gives ...
Atomic Structure and Quantum Theory
... Planck and Blackbody Radiation Einstein and the Photoelectric Effect Spectra Quantization ...
... Planck and Blackbody Radiation Einstein and the Photoelectric Effect Spectra Quantization ...
final2012
... a) What orbitals are filled for the protons and neutrons in these two nuclei? List how many neutrons or protons are in each energy level. Use the notation that lists energy levels by primary quantum number, orbital shell type and total angular momentum, rather than shell number. (Hint – all of the l ...
... a) What orbitals are filled for the protons and neutrons in these two nuclei? List how many neutrons or protons are in each energy level. Use the notation that lists energy levels by primary quantum number, orbital shell type and total angular momentum, rather than shell number. (Hint – all of the l ...
Calculating particle properties of a wave
... 1) Quantum physics is important at small distances d, smaller than the de Broglie wavelength =h/p : d< Example: Nanotechnology for shaping electron waves. 2) Quantum physics is important for large energy quanta E = h f : Example: Planck’s radiation law cuts the spectrum off when the energy to crea ...
... 1) Quantum physics is important at small distances d, smaller than the de Broglie wavelength =h/p : d< Example: Nanotechnology for shaping electron waves. 2) Quantum physics is important for large energy quanta E = h f : Example: Planck’s radiation law cuts the spectrum off when the energy to crea ...
1. a) 25% b)86% 2. For my opinion, I think the way to make
... It is used to determine the absorption light from a sample and it can be used as a detector of HPLC. The concentration of analyst in solution can be determined by measuring the absorbance of single wavelength and applying the Beer-Lambert Law. First, place the sample in the Uv-Vis beam and record th ...
... It is used to determine the absorption light from a sample and it can be used as a detector of HPLC. The concentration of analyst in solution can be determined by measuring the absorbance of single wavelength and applying the Beer-Lambert Law. First, place the sample in the Uv-Vis beam and record th ...
Which scientist developed the quantum mechanical model of the
... Convert this wavelength to centimeters. ...
... Convert this wavelength to centimeters. ...
Physics 12 Assignmen.. - hrsbstaff.ednet.ns.ca
... 3. How can the spectrum of hydrogen contain so many lines when hydrogen contains only one electron? Even though hydrogen only has one electron, it still has an infinite number of energy states for that one electron to occupy and each line in the spectrum represents a transition between two of those ...
... 3. How can the spectrum of hydrogen contain so many lines when hydrogen contains only one electron? Even though hydrogen only has one electron, it still has an infinite number of energy states for that one electron to occupy and each line in the spectrum represents a transition between two of those ...
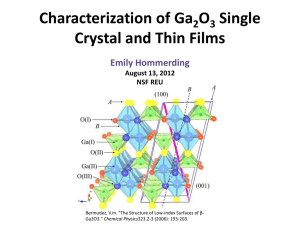
Characterization of Ga 2 0 3 Single Crystal and Thin Films
... The Goal: Gradually sputter away a spot of Pd for a future ...
... The Goal: Gradually sputter away a spot of Pd for a future ...
Lecture 24 (7.1-7.2)
... – The energy profile of the emitted light could not be explained by the classical mechanics which assumes that the energy of an object can be continuously changed – Plank (1900) explained the energy profiles by assuming that the energy of an object can be changed only in discrete amounts (quanta) → ...
... – The energy profile of the emitted light could not be explained by the classical mechanics which assumes that the energy of an object can be continuously changed – Plank (1900) explained the energy profiles by assuming that the energy of an object can be changed only in discrete amounts (quanta) → ...
Pico-Projectors - Broad Shoulder Consulting
... Silicon is widely used down to ~300 nm GaP detectors available down to ~150 nm A wide range of fluorescent materials are available with absorption down to very short wavelength and emission in visible band The problem of UV detection is inherently simpler than IR: ◦ lots of ways to rob a high-energy ...
... Silicon is widely used down to ~300 nm GaP detectors available down to ~150 nm A wide range of fluorescent materials are available with absorption down to very short wavelength and emission in visible band The problem of UV detection is inherently simpler than IR: ◦ lots of ways to rob a high-energy ...
Chapter 27
... • No electrons are emitted if the incident light frequency is below some cutoff frequency that is characteristic of the material being illuminated • The maximum kinetic energy of the photoelectrons is independent of the light intensity • The maximum kinetic energy of the photoelectrons ...
... • No electrons are emitted if the incident light frequency is below some cutoff frequency that is characteristic of the material being illuminated • The maximum kinetic energy of the photoelectrons is independent of the light intensity • The maximum kinetic energy of the photoelectrons ...
The Quantum Mechanical Behavior of Light and Matter
... Spectrum of Hydrogen Atom (cont.) in addition to discrete line transitions, H atom can scatter radiation (propagation direction changes, but E, wavelength unaffected) make transitions to, from continuum at n= 1, λ = 912 Å (Lyman limit) required for ionization photons with λ > 912 Å absorbed only ...
... Spectrum of Hydrogen Atom (cont.) in addition to discrete line transitions, H atom can scatter radiation (propagation direction changes, but E, wavelength unaffected) make transitions to, from continuum at n= 1, λ = 912 Å (Lyman limit) required for ionization photons with λ > 912 Å absorbed only ...
Atomic Spectra Bohr Model Notes
... Photoelectric Effect Looks at the emission of electrons from a metal when light shines on the metal. Light causes electrons to be ejected from the metal. ...
... Photoelectric Effect Looks at the emission of electrons from a metal when light shines on the metal. Light causes electrons to be ejected from the metal. ...
L 35 Modern Physics [1] Modern Physics
... according to classical ideas, should very quickly radiate away all of its energy • If this were so, then we would observe that atoms emit light over a continuous range of wavelengths (colors) NOT SO! ...
... according to classical ideas, should very quickly radiate away all of its energy • If this were so, then we would observe that atoms emit light over a continuous range of wavelengths (colors) NOT SO! ...
X-ray fluorescence

X-ray fluorescence (XRF) is the emission of characteristic ""secondary"" (or fluorescent) X-rays from a material that has been excited by bombarding with high-energy X-rays or gamma rays. The phenomenon is widely used for elemental analysis and chemical analysis, particularly in the investigation of metals, glass, ceramics and building materials, and for research in geochemistry, forensic science and archaeology.
![L 35 Modern Physics [1]](http://s1.studyres.com/store/data/008517000_1-9aef89c0ca089782f518550164188024-300x300.png)

















![L 35 Modern Physics [1] Modern Physics](http://s1.studyres.com/store/data/003926344_1-b779c05b753c6dc3972377c21f9bdcd3-300x300.png)

![L 35 Modern Physics [1]](http://s1.studyres.com/store/data/001689016_1-3e506855e2f70cb00e132a79d00855e2-300x300.png)