atomic emission spectrum
... this frequency into wavelength (nm). Does this frequency fall in the visible region? ...
... this frequency into wavelength (nm). Does this frequency fall in the visible region? ...
Modern physics 2330
... 1- ( ) A black body is defined as an object that absorbs all the electromagnetic radiation falling on it and consequently appears black. 2- ( ) In certain situations light exhibits wave properties like diffraction and Compton Effect. 3- ( ) The photoelectric effect takes place only if the energy of ...
... 1- ( ) A black body is defined as an object that absorbs all the electromagnetic radiation falling on it and consequently appears black. 2- ( ) In certain situations light exhibits wave properties like diffraction and Compton Effect. 3- ( ) The photoelectric effect takes place only if the energy of ...
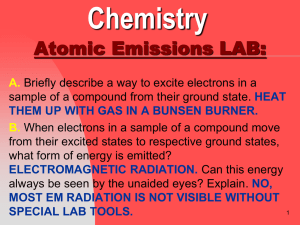
Atomic Emissions LAB Questions
... EACH ELEMENT HAS A UNIQUE SET OF SPECTAL LINES (IS LIKE A FINGER PRINT). F. Why is it possible for a sample of the element hydrogen, in which each atom only has one electron, to have an emission spectrum with more than one color of light? A SAMPLE HAS MANY ATOMS; EACH ELECTRON IN EACH ATOM WILL MOVE ...
... EACH ELEMENT HAS A UNIQUE SET OF SPECTAL LINES (IS LIKE A FINGER PRINT). F. Why is it possible for a sample of the element hydrogen, in which each atom only has one electron, to have an emission spectrum with more than one color of light? A SAMPLE HAS MANY ATOMS; EACH ELECTRON IN EACH ATOM WILL MOVE ...
Slide 1
... Absorption of X-rays on passing through materials depends on the atomic number of the elements Be is the best window, while Pb is a good shielding materials White radiation and unwanted Kb lines can be filtered ...
... Absorption of X-rays on passing through materials depends on the atomic number of the elements Be is the best window, while Pb is a good shielding materials White radiation and unwanted Kb lines can be filtered ...
Electromagnetic Spectrum Wavelength Wavenumber Frequency
... Dative Covalent Bond Coordination number ...
... Dative Covalent Bond Coordination number ...
Introduction to Spectroscopy
... scattering) or at a different frequency (Raman, Brillouin, fluorescence, etc.) or be degraded to heat or initiate a photochemical event or … ...
... scattering) or at a different frequency (Raman, Brillouin, fluorescence, etc.) or be degraded to heat or initiate a photochemical event or … ...
Laboratory Exercise: The Electronic Structure of the Hydrogen Atom
... In this laboratory exercise, we will probe the behavior of electrons within atoms using Emission Spectroscopy. In particular, we will focus on the behavior of the electron in the simplest atom, Hydrogen, and this atom's emission spectrum. For comparison, we will look at the emission spectrum of the ...
... In this laboratory exercise, we will probe the behavior of electrons within atoms using Emission Spectroscopy. In particular, we will focus on the behavior of the electron in the simplest atom, Hydrogen, and this atom's emission spectrum. For comparison, we will look at the emission spectrum of the ...
AP Chemistry Chapter 7 Review Packet
... The orange-yellow color of sodium-vapor streetlights results from electrons in sodium atoms falling from 3p to 3s orbitals. The wavelength of one orange-yellow line in the spectrum of sodium is 589 nm. a. Write the electron configuration for the ground state of sodium. b. Write the electron configur ...
... The orange-yellow color of sodium-vapor streetlights results from electrons in sodium atoms falling from 3p to 3s orbitals. The wavelength of one orange-yellow line in the spectrum of sodium is 589 nm. a. Write the electron configuration for the ground state of sodium. b. Write the electron configur ...
CHM 50- Class activity
... Complete the table of wavelength, frequency and energy values. Wavelength Frequency Energy per photon ...
... Complete the table of wavelength, frequency and energy values. Wavelength Frequency Energy per photon ...
lecture 5 radiation and matter
... Elastic and inelastic events in EM, a beam e- can lose its kinetic energy in many different events, each of these is, at least in part, a quantized transfer ...
... Elastic and inelastic events in EM, a beam e- can lose its kinetic energy in many different events, each of these is, at least in part, a quantized transfer ...
X-ray Diffraction
... The Tel-Atomic x-ray generator, a small shielded x-ray source with a built in diffractometer. Inside there is a power supply and x-ray tube with a copper anode (clearly visible through the glass envelope of the tube). The x-rays are collimated by a lead slit and emerge as a beam toward the crystal m ...
... The Tel-Atomic x-ray generator, a small shielded x-ray source with a built in diffractometer. Inside there is a power supply and x-ray tube with a copper anode (clearly visible through the glass envelope of the tube). The x-rays are collimated by a lead slit and emerge as a beam toward the crystal m ...
Chemistry 1 Concept 5 “Electrons in Atoms” Study Guide
... 2. Specific wavelengths of light seen through a prism that are made when high voltage current is passed through a tube of hydrogen gas at low pressure is _________________ 3. The energy of a photon is related to its ______________ 4. Give the number of orbitals for each sublevel: s _____, p ______, ...
... 2. Specific wavelengths of light seen through a prism that are made when high voltage current is passed through a tube of hydrogen gas at low pressure is _________________ 3. The energy of a photon is related to its ______________ 4. Give the number of orbitals for each sublevel: s _____, p ______, ...
The Photoelectric Effect in Practice WS Key
... kinetic energy __________________________ of the electron. This is equal to the difference between the energy of the photon and the work function __________________________ of the atom. If the light has less energy than that amount, an electron will not be ejected/emitted/released __________________ ...
... kinetic energy __________________________ of the electron. This is equal to the difference between the energy of the photon and the work function __________________________ of the atom. If the light has less energy than that amount, an electron will not be ejected/emitted/released __________________ ...
The length of photon
... The problems of today challenge us to develop more accurate methods of manufacturing and measurements. This trend requires precise determination of fundamental physical quantities. Many manufacturing methods, such as laser processing or photolithography, are based on precisely controlled beam of lig ...
... The problems of today challenge us to develop more accurate methods of manufacturing and measurements. This trend requires precise determination of fundamental physical quantities. Many manufacturing methods, such as laser processing or photolithography, are based on precisely controlled beam of lig ...
Pre-AP Chemistry
... 1. What kind of proportion is shown by the equation c = λ ν? 2. Who stated that electromagnetic radiation has a dual nature? 3. How many meters are 665 nm equal to? 4. What wave measure is identified by the unit Hz? 5. What wave property is known as distance between similar points on a wave? 6. What ...
... 1. What kind of proportion is shown by the equation c = λ ν? 2. Who stated that electromagnetic radiation has a dual nature? 3. How many meters are 665 nm equal to? 4. What wave measure is identified by the unit Hz? 5. What wave property is known as distance between similar points on a wave? 6. What ...
Quiz 4 - El Camino College
... would it appear? If not visible, what region of the electromagnetic spectrum would it be in? ...
... would it appear? If not visible, what region of the electromagnetic spectrum would it be in? ...
Free-electron lasers
... The mechanism is understood now better and could help to make synthetic catalysts. ...
... The mechanism is understood now better and could help to make synthetic catalysts. ...
Unit 2 Intro Worksheet - Coral Gables Senior High
... 2. states the impossibility of knowing both velocity and position of a moving particle at the same time ...
... 2. states the impossibility of knowing both velocity and position of a moving particle at the same time ...
151b650e7a25cfd
... absorbed in the crystal. The number of light photons produced, m, might be about 1,000 for a typical scintillation crystal. A typical crystal might have an optical efficiency, k, of 0.5 - in other words 50% of the light produced reaches the photocathode which might have a quantum efficiency of 0.15. ...
... absorbed in the crystal. The number of light photons produced, m, might be about 1,000 for a typical scintillation crystal. A typical crystal might have an optical efficiency, k, of 0.5 - in other words 50% of the light produced reaches the photocathode which might have a quantum efficiency of 0.15. ...
Set 3
... 1) Suppose that a 60 W light bulb radiates primarily at a wavelength 1000 nm. What is the number of photons emitted per second ? 2) What is the number of photons emitted per second by the Sun? Lets assume that the star radiates electromagnetic waves with λ = 550 nm and its power equals to 3.9 · 1026 ...
... 1) Suppose that a 60 W light bulb radiates primarily at a wavelength 1000 nm. What is the number of photons emitted per second ? 2) What is the number of photons emitted per second by the Sun? Lets assume that the star radiates electromagnetic waves with λ = 550 nm and its power equals to 3.9 · 1026 ...
here
... •Earth’s atmosphere absorbs and reflects radiation at several wavelengths •From the ground, we only detect visible and radio •For other wavelengths, we must observe in a place above most or all of the atmosphere: Mountains: Near IR Planes: Far IR Balloons: UV, X-ray Space: everything including gamma ...
... •Earth’s atmosphere absorbs and reflects radiation at several wavelengths •From the ground, we only detect visible and radio •For other wavelengths, we must observe in a place above most or all of the atmosphere: Mountains: Near IR Planes: Far IR Balloons: UV, X-ray Space: everything including gamma ...
The end
... b/ One milliwatt of light of wavelength 4,560A is incident on a caesium surface. Calculate the electron current liberated and the minimum stopping voltage necessary to reduce this current to zero. Work function of caesium is 1.93 volts. Assume a quantum efficiency of 0.5 % (that means only 0,5 % of ...
... b/ One milliwatt of light of wavelength 4,560A is incident on a caesium surface. Calculate the electron current liberated and the minimum stopping voltage necessary to reduce this current to zero. Work function of caesium is 1.93 volts. Assume a quantum efficiency of 0.5 % (that means only 0,5 % of ...
X-ray fluorescence

X-ray fluorescence (XRF) is the emission of characteristic ""secondary"" (or fluorescent) X-rays from a material that has been excited by bombarding with high-energy X-rays or gamma rays. The phenomenon is widely used for elemental analysis and chemical analysis, particularly in the investigation of metals, glass, ceramics and building materials, and for research in geochemistry, forensic science and archaeology.