Bohr Model and EMS practice
... wavelength of energy is emitted? What region of the EM spectrum is this wavelength located? 2. In what region of the EM spectrum is energy emitted when an electron moves from n=5 to n=3? 3. When an electron in an excited state moves from n=4 to n=1, what wavelength of energy is emitted? In what regi ...
... wavelength of energy is emitted? What region of the EM spectrum is this wavelength located? 2. In what region of the EM spectrum is energy emitted when an electron moves from n=5 to n=3? 3. When an electron in an excited state moves from n=4 to n=1, what wavelength of energy is emitted? In what regi ...
الشريحة 1
... Effect of Scattered Radiation at Wavelength Extremes of an Instrument Wavelength extremes of an instrument are dependent on type of source, detector and optical components used in the manufacture of the instrument. Outside the working range of the instrument, it is not possible to use it for accura ...
... Effect of Scattered Radiation at Wavelength Extremes of an Instrument Wavelength extremes of an instrument are dependent on type of source, detector and optical components used in the manufacture of the instrument. Outside the working range of the instrument, it is not possible to use it for accura ...
Poster - Research
... Ultrathin metal films grown on semiconductor substrates exhibit a rich variety of growth behavior and morphology at different temperatures due to quantum size effects. We have used the surface x-ray diffraction station at Sector 33ID (UNICAT) to study the nanoscale structural evolution of Pb films g ...
... Ultrathin metal films grown on semiconductor substrates exhibit a rich variety of growth behavior and morphology at different temperatures due to quantum size effects. We have used the surface x-ray diffraction station at Sector 33ID (UNICAT) to study the nanoscale structural evolution of Pb films g ...
Review - ND
... an experiment using thin gold foil and a beam of alpha particles. One of the observations was that some alpha particles scattered back from the foil towards the source. i) Describe Rutherford's model of the atom. ii) How does this model explain the experimental observation that some of the alpha par ...
... an experiment using thin gold foil and a beam of alpha particles. One of the observations was that some alpha particles scattered back from the foil towards the source. i) Describe Rutherford's model of the atom. ii) How does this model explain the experimental observation that some of the alpha par ...
The birth of quantum mechanics
... The power radiated in wavelength interval dl is proportional to the fraction of a wavelength: dP ~ dl/l So the energy density (J/m3) is ...
... The power radiated in wavelength interval dl is proportional to the fraction of a wavelength: dP ~ dl/l So the energy density (J/m3) is ...
Electron configuration of atoms
... • When a Hydrogen atom is in the ground state, n=1, it does not radiate any energy. • When energy is added from an external source, the electron moves to a higher energy orbit. • The atom is now in an excited state. • The electron then drops to a lower state and emits a photon corresponding to the d ...
... • When a Hydrogen atom is in the ground state, n=1, it does not radiate any energy. • When energy is added from an external source, the electron moves to a higher energy orbit. • The atom is now in an excited state. • The electron then drops to a lower state and emits a photon corresponding to the d ...
photon particle - wave duality
... for the measurement process because of the way wave-packets are constructed. It turns out that the smaller the wave-packet (the more localized the photon), the larger the spread of wavelengths needed to construct the packet. This implies that both the wavelength and the position of a photon cannot b ...
... for the measurement process because of the way wave-packets are constructed. It turns out that the smaller the wave-packet (the more localized the photon), the larger the spread of wavelengths needed to construct the packet. This implies that both the wavelength and the position of a photon cannot b ...
Chapter 3 Electromagnetic Theory, Photons, and Light
... 1900: to explain black body radiation spectrum Max Planck suggested that light is emitted in small indivisible quanta of energy: E=h (h=6.626×10-34 J.s) 1905: to explain photoelectric effect Einstein stated that EM field itself is quantized Photons cannot be observed directly, one can only see them ...
... 1900: to explain black body radiation spectrum Max Planck suggested that light is emitted in small indivisible quanta of energy: E=h (h=6.626×10-34 J.s) 1905: to explain photoelectric effect Einstein stated that EM field itself is quantized Photons cannot be observed directly, one can only see them ...
Quantum Mechanical Model of the Atom and Electronic Structure 1
... For each principal energy level designated by the principal quantum number n (n = 1, 2, 3, ...) there are n sublevels. The sublevels are characterized by the angular momentum quantum number l and are designated s, p, d, f, g ... For a given value of n, sublevel energy varies s < p < d < f ... ...
... For each principal energy level designated by the principal quantum number n (n = 1, 2, 3, ...) there are n sublevels. The sublevels are characterized by the angular momentum quantum number l and are designated s, p, d, f, g ... For a given value of n, sublevel energy varies s < p < d < f ... ...
Tutorial 5 - Electrical and Computer Engineering
... – If this energy is bigger than the bandgap of a detector material, e-h pairs will be created – IR has lower energies than visible, so the bandgap has to be reduced – Detector bandgaps can be tuned from 0eV up – These detectors must operate at very low temperatures • Restricted to special uses ...
... – If this energy is bigger than the bandgap of a detector material, e-h pairs will be created – IR has lower energies than visible, so the bandgap has to be reduced – Detector bandgaps can be tuned from 0eV up – These detectors must operate at very low temperatures • Restricted to special uses ...
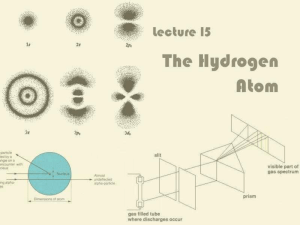
Lecture 15: The Hydrogen Atom
... He found that, once in a while, the a-particles were scattered backwards by the target ...
... He found that, once in a while, the a-particles were scattered backwards by the target ...
Activity 2 - hrsbstaff.ednet.ns.ca
... 3. A standard He-Ne laser produces about 1.0 mW of red light at a wavelength of 633 nm. To create a single-photon interference experiment the laser is shone through a series of filters that reduce the beam to a small fraction of the original number of photons. (a) Calculate the number of photons pro ...
... 3. A standard He-Ne laser produces about 1.0 mW of red light at a wavelength of 633 nm. To create a single-photon interference experiment the laser is shone through a series of filters that reduce the beam to a small fraction of the original number of photons. (a) Calculate the number of photons pro ...
Spectroscopy - Birmingham City Schools
... Light Light is electromagnetic radiation (abbr. EM) The light we see is only a small portion of the electromagnetic spectrum Isaac Newton (1400’s) discovered that white light is made up of many different colors. 1801, Thomas Young performed slit experiment that showed light was a wave Crest ...
... Light Light is electromagnetic radiation (abbr. EM) The light we see is only a small portion of the electromagnetic spectrum Isaac Newton (1400’s) discovered that white light is made up of many different colors. 1801, Thomas Young performed slit experiment that showed light was a wave Crest ...
Blackbody Radiation and Planck`s Hypothesis of Quantized Energy
... Photons and the Photoelectric Effect Classical predictions: 1. Any beam of light of any color can eject electrons if it is intense enough. 2. The maximum kinetic energy of an ejected electron should increase as the intensity increases. Observations: 1. Light must have a certain minimum frequency in ...
... Photons and the Photoelectric Effect Classical predictions: 1. Any beam of light of any color can eject electrons if it is intense enough. 2. The maximum kinetic energy of an ejected electron should increase as the intensity increases. Observations: 1. Light must have a certain minimum frequency in ...
Module 1 - Identifying Metals Using Atomic Emission
... high voltage is applied across the anode and cathode, resulting in an ionization of the fill gas. The gas ions are accelerated towards the cathode and, upon impact on the cathode, sputter cathode material that is excited in the glow discharge to emit the radiation of the sputtered material (i.e., th ...
... high voltage is applied across the anode and cathode, resulting in an ionization of the fill gas. The gas ions are accelerated towards the cathode and, upon impact on the cathode, sputter cathode material that is excited in the glow discharge to emit the radiation of the sputtered material (i.e., th ...
Lecture 27: Quantum Physics
... electromagnetic waves with very short wavelengths (~0.1 nm), it should be possible to diffract them using the regular atomic spacings of crystal lattice as a diffraction grating. • Experiments demonstrated that his suggestion was indeed valid. ...
... electromagnetic waves with very short wavelengths (~0.1 nm), it should be possible to diffract them using the regular atomic spacings of crystal lattice as a diffraction grating. • Experiments demonstrated that his suggestion was indeed valid. ...
Atomic Theory Study Guide - Reading Community Schools
... Equation, and identify which orbital properties are determined by each of these numbers. 2. Name orbitals given its quantum numbers, or identify quantum numbers for given orbital. 3. Sketch the relative shapes, sizes, and spatial orientations of s, p, and d orbitals of the hydrogen atom. 4. Apply th ...
... Equation, and identify which orbital properties are determined by each of these numbers. 2. Name orbitals given its quantum numbers, or identify quantum numbers for given orbital. 3. Sketch the relative shapes, sizes, and spatial orientations of s, p, and d orbitals of the hydrogen atom. 4. Apply th ...
electromagnetic spectrum and flame tests
... When investigators passed an electric current through a vacuum tube containing hydrogen gas at low pressure, they observed the emission of a characteristic pinkish glow. When a narrow beam of the emitted light was shined through a prism, it was separated into a series of specific frequencies (and th ...
... When investigators passed an electric current through a vacuum tube containing hydrogen gas at low pressure, they observed the emission of a characteristic pinkish glow. When a narrow beam of the emitted light was shined through a prism, it was separated into a series of specific frequencies (and th ...
Questions and Answers - hrsbstaff.ednet.ns.ca
... 1. Which of the coloured lights (red, orange, blue) on a Christmas tree emits photons with the most and least energy? Explain. 2. Does your stove emit energy when the burner is not turned on? Explain. 3. A single photon is ejected from a light source with a frequency of 2.0 x 1014 Hz. How much energ ...
... 1. Which of the coloured lights (red, orange, blue) on a Christmas tree emits photons with the most and least energy? Explain. 2. Does your stove emit energy when the burner is not turned on? Explain. 3. A single photon is ejected from a light source with a frequency of 2.0 x 1014 Hz. How much energ ...
X-ray fluorescence

X-ray fluorescence (XRF) is the emission of characteristic ""secondary"" (or fluorescent) X-rays from a material that has been excited by bombarding with high-energy X-rays or gamma rays. The phenomenon is widely used for elemental analysis and chemical analysis, particularly in the investigation of metals, glass, ceramics and building materials, and for research in geochemistry, forensic science and archaeology.