* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download File
Double-slit experiment wikipedia , lookup
Elementary particle wikipedia , lookup
Bremsstrahlung wikipedia , lookup
James Franck wikipedia , lookup
Renormalization wikipedia , lookup
Delayed choice quantum eraser wikipedia , lookup
Particle in a box wikipedia , lookup
Tight binding wikipedia , lookup
X-ray photoelectron spectroscopy wikipedia , lookup
Quantum electrodynamics wikipedia , lookup
Matter wave wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Atomic orbital wikipedia , lookup
Bohr–Einstein debates wikipedia , lookup
X-ray fluorescence wikipedia , lookup
Electron scattering wikipedia , lookup
Hydrogen atom wikipedia , lookup
Electron configuration wikipedia , lookup
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
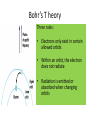
Bohr’s Model of the Atom Chapter 5 • The Modern • The atom is mostly View of the empty space. Atom • Two regions 1. The Nucleus – Protons and neutrons are located in the nucleus. 2. Electron cloud region – Place where you might find an electron. • The Atom is • From his measurements, Rutherford estimated the mostly radius of an atom to be empty space 100,000 times greater than the radius of the nucleus. This is comparable to the thickness of a dime to the length of football field Max Planck’s Quantum Theory • What did • Atom energy is Planck absorbed or proposed? liberated in packets or chunks of energy. • Quanta = Planck called these plural packages of energy • Quantum = "quanta". singular • Photon • The Quantum of Light = the Photon. • Particles of light are called light quanta or photons. Energy of a Photon = h x u E = h x u or E = h x f h is Planck’s constant h= 6.63 x 10-34 J.s u is equal to the frequency of light. u = f = frequency Packet of energy in a photon is so small that we are not aware of the rain of photons of light impinging on our eyes – just as you cannot feel the impact of individual air molecules. The quantum concept • Max Planck (1900) – Introduced quantized energy • Einstein (1905) – Light made up of quantized photons • Higher frequency photons = more energetic photons Bohr’s atomic model • Niels Bohr puts it all together • Bohr added Planck’s quanta idea (which was proven by Einstein and Louis de Broglie’s wave/particle theory) to the Rutherford’s atomic model. • Electrons exist at set levels of energy, at fixed distances from the nucleus. • When an atom absorbs energy, the electron jumps to a level further from the nucleus • If it radiated energy, that means that the electron is falling to a level closer to the nucleus. • The protons and neutrons are located in the nucleus. • The electrons are located at different energy shells (energy levels around the nucleus) Energy Levels/shells • There are several energy levels in which an electron might be found. ee- • To move from one level to another an electron must gain or lose an exact amount of energy. Quantum ee- 2 electron Bohr’s T heory Three rules: • Electrons only exist in certain allowed orbits • Within an orbit, the electron does not radiate • Radiation is emitted or absorbed when changing orbits