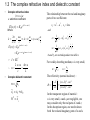* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Optical Properties of Condensed Matters
Dispersion staining wikipedia , lookup
Nitrogen-vacancy center wikipedia , lookup
Nonimaging optics wikipedia , lookup
Fiber-optic communication wikipedia , lookup
Optical rogue waves wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Atmospheric optics wikipedia , lookup
Franck–Condon principle wikipedia , lookup
Surface plasmon resonance microscopy wikipedia , lookup
Optical amplifier wikipedia , lookup
Ultrafast laser spectroscopy wikipedia , lookup
Ellipsometry wikipedia , lookup
Upconverting nanoparticles wikipedia , lookup
Birefringence wikipedia , lookup
Photon scanning microscopy wikipedia , lookup
Anti-reflective coating wikipedia , lookup
Astronomical spectroscopy wikipedia , lookup
Retroreflector wikipedia , lookup
Optical coherence tomography wikipedia , lookup
Harold Hopkins (physicist) wikipedia , lookup
Passive optical network wikipedia , lookup
Silicon photonics wikipedia , lookup
Optical tweezers wikipedia , lookup
3D optical data storage wikipedia , lookup
Nonlinear optics wikipedia , lookup
X-ray fluorescence wikipedia , lookup
Transparent ceramics wikipedia , lookup
Ultraviolet–visible spectroscopy wikipedia , lookup
Optical Properties of Condensed Matters 祝 世宁 引言: 光学过程的分类,光学系数,复折射率与复介电常数,光学材料(绝缘晶体,半导 体,玻璃,金属,高分子材料等),凝聚态物质光学性质的特征(对称性,电子能 带,晶格振动,态密度,局域态和集体激发等),微观模型 光在凝聚态物质中传播的经典理论: 光在稠密光学介质中的传播,偶极振子模型,色散理 论,光学各向异性:双折射 带间吸收: 带间跃迁, 直接跃迁的跃迁几率,直接带隙半导体的带边吸收,间接带隙半导 体的带边吸收,带边以上的带间吸收,吸收谱的测量,光探测材料与器件 激子: 激子的概念,自由激子,外场中的自由激子,高密度的自由激子,弗仑克尔激子 发光: 固体中光的发射,带间发光,光致发光,电致发光 量子阱与量子点: 量子限制结构,半导体量子阱的结构与制备,电子能级,光的吸收与激 发,量子限制斯塔克效应,光发射,量子阱子的带间跃迁,Bloch振子,量子点 自由电子: Plasma反射率, 自由载流子电导,金属,掺杂半导体,Plasmon 高分子材料: 高分子材料简介,共轭分子的电子态,高分子光谱,芳烃共轭聚合物,有机 光电子学 发光中心: 电子—声子相互作用,色心,离子晶体中的顺磁杂质,固体激光器与放大器, 发光材料 声子: 红外活性声子,极性晶体红外反射与吸收,极化激元,极化子,非弹性光散射,声 子寿命 非线性光学: 非线性极化率张量,光学非线性的物理起源,二阶非线性效应,三阶非线性 效应 光子晶体和光学微腔: 光子能带,光子晶体的构成,光学微腔,腔量子电动力学简介 Chapter 1 Introduction 1.1 Classification of optical processes • Reflection refractive index n() = c / v () • Propagation • Transmission Snell’s law absorption ~ resonance luminescence ~ spontaneous emission Optical medium elastic and Inelastic scattering nonlinear-optics Optical medium Propagation 1.2 Optical coefficients • Coefficient of reflection or reflectivity (R): R = reflected power / incident power • Transmission or transmissivity (T): T = transmitted power / incident power • Luminescence R+T=1 • Refractive index (n): • Absorption coefficient () = - d I * d z / I (z); Beer’s law: I ( z ) I 0 e z is strong function of frequency The atom jumps to an excited state by absorption of a Photon, then relaxes to an intermediate state, before reemitting a photon by spontaneous emission as it falls To the ground state. The photon emitted has a smaller energy than the absorbed photon. The reduction in the Photon energy is called the Stokes shift. • Scattering Variation of n of the medium on a length scale smaller than the of the light I ( z ) I 0 exp( N s z ) N: the number of scattering centres / V; S: scattering cross-section; = N S T (1 R1 )e l (1 R2 ) (1 R) 2 e l Rayleigh scattering : s ( ) ~ 1 4 1.3 The complex refractive index and dielectric constant • Complex refractive index ~ n i n : extinction coefficient E ( z , t ) E0 e i ( k z t ) Where k 2 n ~ ( n i ) k n /n c c c ~ E ( z , t ) E0 e i ( n z / c t ) E0 e w / c e i ( n z / c t ) I EE * 2 4 c • Complex dielectric constant n r ~ i r 1 n~ 2 ~r 2 The relationship between the real and imaginary parts of two coefficients: 1 n 2 2 , 2 2n and 1 1 1 n (1 (12 22 ) 2 ) 2 2 1 1 1 (1 (12 22 ) 2 ) 2 . 2 ~ and ~ n r are not independent var iable s For weakly absorbing medium, is very small, n 1 , 2 2n The reflectivity (normal incidence) : ~ 1 2 ( n 1) 2 2 n R ~ . n 1 ( n 1) 2 2 In the transparent region of material : is very small, and 2 are negligible, one may consider only the real parts of n and ; In the absorption region, one need to know both the real and imaginary parts of n and . 1.4 Optical materials 1.41 Crystalline insulators and semiconductors Transparency range, the index may be taken to be real with no imaginary component (approximately constant n=1.77) R = 0.077, hence T =(1-R)2=0.85 Phonon absorption or lattice absorption Due to absorption by bound electrons Fundamental absorption edge, is determined by the band gap. The optical properties of semiconductors are similar to those of insulators, expect that the electronic and phonic transitions occur at longer wavelengths. Its transparency range lies outside the visible spectrum, so it has a dark Metallic appearance. 1.4 Optical materials 1.41 Crystalline insulators and semiconductors Materials can take on new properties by controlled doping with optically active substance. The principle of doping optically active atoms into colourless hosts is employed extensively in the crystals used for solid state lasers. A typical example is the ruby crystal. Rubies consist of Cr+3 ions doped into Al2O3. In the natural crystals, the Cr+3 ions are present as impurities, but in synthetic crystals, the dopants are deliberately introduced in controlled quantities during the crystal growth process. Transmission spectrum of ruby (ruby Al2O3 With 0.05% Cr3+) compared to sapphire(pure Al2O3). The thicknesses of the two crystals were 6.1 mm and 3.0 mm, respectively 1.4 Optical materials 1.4.2 Glass • • • • Most types of glasses are made of silica (SiO2) with other chemicals. Insulator, all the characteristic features crystalline insulators, the trans range from around 200 nm to beyond 2000 nm; Small absorption and scattering losses; n changes by less than 1% over the whole visible spectral region; Chemicals are commonly added to silica during the fusion process to alter the refractive index and transmission range; Stained glass and colour glass filter are made by adding semiconductors with gaps in visible spectral region. 1.4 Optical materials 1.4.3 Metals • • • Reflectivity of silver from the infrared to the ultraviolet Reflect infrared and visible, but transmit ultraviolet (ultraviolet transmission of metals); High reflectivity is caused by the interaction of the light with the free electrons in metal; There is a characteristic cut-off frequency called the plasma frequency. 1.4 Optical materials 1.4.4 Molecular (large organic molecules) Materials • • • The molecular materials are held together by the weak van de Waals bonds, whereas the molecules are held together by strong covalent bonds. The optical properties of materials are similar to those of the individual molecules; Saturated compounds: compounds which do not contain any free valence (all the electrons are tightly held in their bonds), and are transparent in the visible, absorb in the infrared and ultraviolet (insulator crystals); Conjugated molecules (bezene C6H6): The electrons form large delocalized orbitals called orbitals which spread out across the whole molecule, therefore are less tightly bound than the electrons in saturated molecules. The molecules with visible absorption also tend to emit strongly at visible frequencies (semiconductors); Absorption spectrum of the polyfluorene-based polymers F8. Conjugated polymers such as F8 luminesce strongly When electrons are promoted into the excited states of the molecule. The luminescence is Stokes shifted to lower energy compared to absorption, and typically occurs in the middle of the visible spectral region. The emission wavelength can be tuned by small alternation to the chemical structure of the molecular unit within the polymers. The property has been used to develop organic light emitting devices to cover the full visible spectral region. 1.5 Characteristic optical physics in the condensed matter What the difference is between condensed matter and atomic or molecular optical physics? • Crystal symmetry • • • • Electronic bands Vibronic bands The density of states Delocalized states and collective excitations 1.5.1 Crystal symmetry * long range translational order Electronic bands, delocalized states, … … * point group symmetry Neumann’s principle The measurable property point group symmetry Any macroscopic physical property must have at least the symmetry of the crystal structure Optical anisotropy: birefringence, nonlinear optical coefficient … … 1.5 Characteristic optical physics in the condensed matter 1.5.1 Crystal symmetry Optical anisotropy: Lifting of degeneracies: Degeneracy can be lifted by reduction of the symmetry Splitting of the energy levels of a free atom by the crystal field effect determined by the symmetry class of the crystal. The splitting Is caused by the interaction of the orbitals of The atoms with the electric fields of the crystalline environment. Optical transition between these crystal-field spilt levels ofen occur in the visible region, and cause the material to have every interesting properties that are found in the free atoms. 1.5 Characteristic optical physics in the condensed matter 1.5.2 Electronic bands The electronic states within the bands are delocalized and possess the translational invariance of the crystal. Bloch’s theorem states that the wave functions: k (r ) uk (r ) exp( ik r ) where uk(r ) is a function that has the periodicity of the lattice. Each electronic band has a different envelope function As the atoms are brought closer together to form the solid, their outer orbitals begin to overlap with each other. These overlapping orbitals interact strongly, and broad bands are formed. uk(r ) 1.5.3 Vibronic bands The band arises from the coupling of discrete electronic state to a continuous spectrum of vibrational mode. This contrast with the electronic band that arises from interaction between electronic states of neighbouring atoms. 1.5 Characteristic optical physics in the condensed matter 1.5.4 The density of states This is defined as: Number of states in the range E ( E + d E) = g (E) d E. g(E) is work out in practice: g(E) = g(k)·dk / dE This can be evaluated from knowledge of the E-k relationship for electrons or phonons. 1.5.5 Delocalized states and collective excitations phonon: the collective excitation of lattice vibration; exciton: formed from delocalized electrons and holes in semiconductors; plasmon: formed from free electrons in metals and doped semiconductors; …… many optical effects related to these … … 1.5.6 Microscopic models Classical : Treat both the medium and the light according to classical physics; Semiclassical: apply quantum mechanics to atoms, treat light as a classical electromagnetic wave; Fully quantum: both atoms and light are treated quantum mechanically. Exercises: 1. The complex refractive index of germanium at ~ 4.141 i2.215 . Calculate 400 nm is given by n for germanium at 400 nm: (a) the phase velocity of light, (b) the absorption coefficient, and ( c) the reflectivity. 2. Show that the optical density (O.D.) of a sample is related to its transmission T and reflectively R through: O.D. log 10 (T ) 2 log 10 (1 R). Hence explain how you would determine the optical density by making two transmission measurements, one at wavelength where the material absorbs, and the other at a wavelength ’ where the material is transparent.