Lecture 9
... is computed like ray-tracing and the indirect illumination is computed from querying the stored photons in the photon ...
... is computed like ray-tracing and the indirect illumination is computed from querying the stored photons in the photon ...
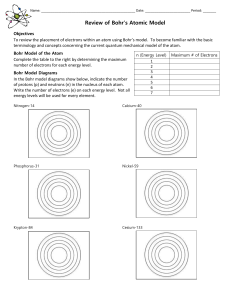
Name: ______ Date: Period: ______ Review of Bohr`s Atomic Model
... To review the placement of electrons within an atom using Bohr’s model. To become familiar with the basic terminology and concepts concerning the current quantum mechanical model of the atom. Bohr Model of the Atom ...
... To review the placement of electrons within an atom using Bohr’s model. To become familiar with the basic terminology and concepts concerning the current quantum mechanical model of the atom. Bohr Model of the Atom ...
Review Notes - Biochemistry
... The goal of all atoms is to have a _STABLE_ outer energy level. The goal leads to bonding of atoms. 2 types of bonding: 1. Ionic Bonding: When _1_ or more electrons are _TRANSFERRED_ from one atom to another. Ion: an atom with a_CHARGE_. When an electron is gained it will be _NEGATIVE_charged ...
... The goal of all atoms is to have a _STABLE_ outer energy level. The goal leads to bonding of atoms. 2 types of bonding: 1. Ionic Bonding: When _1_ or more electrons are _TRANSFERRED_ from one atom to another. Ion: an atom with a_CHARGE_. When an electron is gained it will be _NEGATIVE_charged ...
1s 2s 2p - Solon City Schools
... The photoelectron spectrum is a plot of the number of electrons emitted versus their kinetic energy. In the diagram below, the “X” axis is labeled high to low energies so that you think about the XY intersect as being the nucleus. ...
... The photoelectron spectrum is a plot of the number of electrons emitted versus their kinetic energy. In the diagram below, the “X” axis is labeled high to low energies so that you think about the XY intersect as being the nucleus. ...
Atomic Structure I. History A. prehistory The four elements B
... Matter is composed of extremely small particles called atoms ...
... Matter is composed of extremely small particles called atoms ...
calibration of the advanced ligo detectors for the discovery of the
... Block diagram of the differential arm length feedback control loop. mirror of each detector. The laser The sensing function, digital filter function, and actuation function varies in power, causing a variation in combine to form the open loop transfer function. the force (from the recoil of photons) ...
... Block diagram of the differential arm length feedback control loop. mirror of each detector. The laser The sensing function, digital filter function, and actuation function varies in power, causing a variation in combine to form the open loop transfer function. the force (from the recoil of photons) ...
Excitation of Quantum Jumps by Collisions
... Muonic atoms are objects of the nuclear physics research. ...
... Muonic atoms are objects of the nuclear physics research. ...
SPS 3
... (photons). From this model, it is evident that if only one photon is incident on the beam splitter, then it cannot be simultaneously detected at both the detectors. In that case the joint probability of equal time photo detection at the two detectors will be zero. In this experiment we use single em ...
... (photons). From this model, it is evident that if only one photon is incident on the beam splitter, then it cannot be simultaneously detected at both the detectors. In that case the joint probability of equal time photo detection at the two detectors will be zero. In this experiment we use single em ...
Simple Harmonic Oscillator
... Never express yourself more clearly than you are able to think. Prediction is very difficult, especially about the future. - Niels Bohr ...
... Never express yourself more clearly than you are able to think. Prediction is very difficult, especially about the future. - Niels Bohr ...
Quantum Notes (Chapter 16)(Powerpoint document)
... The wave-particle duality…light can have wave properties in some circumstances (diffraction, interference) and particle properties in others (photons and quantization). ...
... The wave-particle duality…light can have wave properties in some circumstances (diffraction, interference) and particle properties in others (photons and quantization). ...
Chapter 7 Handout 1 Atomic Orbitals Quantum Numbers: Principal
... Rules for filling orbitals: 1. Aufbau Principle: a. Electrons fill up orbitals of lowest energy first b. Orbitals in the same sublevel are equal in energy c. Sometimes energy levels overlap 2. Pauli Exculsion Principle a. There is a max of 2 electrons in any one orbital b. These 2 electrons must ha ...
... Rules for filling orbitals: 1. Aufbau Principle: a. Electrons fill up orbitals of lowest energy first b. Orbitals in the same sublevel are equal in energy c. Sometimes energy levels overlap 2. Pauli Exculsion Principle a. There is a max of 2 electrons in any one orbital b. These 2 electrons must ha ...
Notes on Quantum Theory
... Joules/s) of red light with wavelength 663 nm. Each photon has energy ...
... Joules/s) of red light with wavelength 663 nm. Each photon has energy ...
Experimental proposal for electromagnetically
... crystals that the boundaries between these small crystals scatter a portion of the light reflected from the printed page, which makes this material optically translucent. And finally, the specimen on the right is composed not only of many small, interconnected crystals, but also of a large number of ...
... crystals that the boundaries between these small crystals scatter a portion of the light reflected from the printed page, which makes this material optically translucent. And finally, the specimen on the right is composed not only of many small, interconnected crystals, but also of a large number of ...
Quantum Mechanics Course essay Quantum mechanics Origins of
... • For shorter wavelength’s even 1 photon is too much energy, so shortest wavelengths have very ...
... • For shorter wavelength’s even 1 photon is too much energy, so shortest wavelengths have very ...
Modern Atomic Theory Notes Sheet
... light is passed through a prism the colors that appear are known as the element’s: Light as a Particle and Emission Spectra: Every element has a unique: The atomic emission spectra phenomenon is a key piece of evidence that light acts like a ...
... light is passed through a prism the colors that appear are known as the element’s: Light as a Particle and Emission Spectra: Every element has a unique: The atomic emission spectra phenomenon is a key piece of evidence that light acts like a ...
Light
... energy in small specific amounts (quanta) Quantum – minimum amount of energy that can be gained or lost by an atom ...
... energy in small specific amounts (quanta) Quantum – minimum amount of energy that can be gained or lost by an atom ...
Atomic Structure
... 1. The Bohr model of the atom was the first quantum mechanical model of the atom. a. Bohr postulated that a hydrogen atom could only exist without radiating in one of a set of stationary states. Explain what is meant by this postulate. b. Bohr related his postulate to the classical picture of a hydr ...
... 1. The Bohr model of the atom was the first quantum mechanical model of the atom. a. Bohr postulated that a hydrogen atom could only exist without radiating in one of a set of stationary states. Explain what is meant by this postulate. b. Bohr related his postulate to the classical picture of a hydr ...
New Type of Einstein-Podolsky-Rosen
... m and K are the frequency and the wave vector of the incident beam, and co~, coq and K~, Kq are the frequencies and the wave vectors of the generated light quanta. The desired photon pair can be produced by cutting the crystal at the desired phase-matching angle. The crystal used in the experiment w ...
... m and K are the frequency and the wave vector of the incident beam, and co~, coq and K~, Kq are the frequencies and the wave vectors of the generated light quanta. The desired photon pair can be produced by cutting the crystal at the desired phase-matching angle. The crystal used in the experiment w ...
Worksheet 1 Answer Key from 2010
... degenerate. In short, degenerate means energetically equivalent. 18. Calculate the energy (E) of a neutron in a 1-dimensional "box" of length (L) 200 nm at principle energy level (n) 2. What is the proprtionality of E to n for the 1-dimensional box? E = (n2·h2)/(8·m·L2) E = (22·6.63 E-342)/(8·1.67 E ...
... degenerate. In short, degenerate means energetically equivalent. 18. Calculate the energy (E) of a neutron in a 1-dimensional "box" of length (L) 200 nm at principle energy level (n) 2. What is the proprtionality of E to n for the 1-dimensional box? E = (n2·h2)/(8·m·L2) E = (22·6.63 E-342)/(8·1.67 E ...
UNIT 7 ATOMIC AND NUCLEAR PHYSICS
... E f is the energy of the final state, and Ei is the energy of the initial state. The spectrum of light emitted by electrons making transitions from high to low energy states is called an emission spectrum. An electron in a low energy state could make a transition to a higher energy ...
... E f is the energy of the final state, and Ei is the energy of the initial state. The spectrum of light emitted by electrons making transitions from high to low energy states is called an emission spectrum. An electron in a low energy state could make a transition to a higher energy ...
X-ray fluorescence

X-ray fluorescence (XRF) is the emission of characteristic ""secondary"" (or fluorescent) X-rays from a material that has been excited by bombarding with high-energy X-rays or gamma rays. The phenomenon is widely used for elemental analysis and chemical analysis, particularly in the investigation of metals, glass, ceramics and building materials, and for research in geochemistry, forensic science and archaeology.