Document
... Redox reaction- oxidation , reduction, oxidizing agent, reducing agent Fig. 9.4 Electron carriers- NAD+ is reduced (gains electrons to form NADH “currency in the form of a check”) Fig. 9.5 An introduction to the Electron Transport Chain (NADH will be oxidized “cashed”- has a lot of potential energy ...
... Redox reaction- oxidation , reduction, oxidizing agent, reducing agent Fig. 9.4 Electron carriers- NAD+ is reduced (gains electrons to form NADH “currency in the form of a check”) Fig. 9.5 An introduction to the Electron Transport Chain (NADH will be oxidized “cashed”- has a lot of potential energy ...
Macromolecules, Chemical Reactions & Enzymes
... A catalyst speeds up the rate of a chemical reaction. An enzyme is a catalyst for a biological chemical reaction—inside cells! Enzymes are very specific—one enzyme for one chemical reaction. ...
... A catalyst speeds up the rate of a chemical reaction. An enzyme is a catalyst for a biological chemical reaction—inside cells! Enzymes are very specific—one enzyme for one chemical reaction. ...
Final Exam - UC Davis Plant Sciences
... Dephosphorylation of the PFK-2/F2,6BPase isoenzyme in the liver results in the activation of its kinase activity. Briefly explain the logic of this regulatory loop with respect to the degradation of excess dietary xylulose in the liver. (4 pts) ...
... Dephosphorylation of the PFK-2/F2,6BPase isoenzyme in the liver results in the activation of its kinase activity. Briefly explain the logic of this regulatory loop with respect to the degradation of excess dietary xylulose in the liver. (4 pts) ...
Review for Final Summer 2010
... Where does each reaction take place? (see your worksheet) o Glycolysis o Formation of acetyl CoA o Krebs cycle o Electron transport chain o Fermentation Glycolysis splits sugar to make ATP & NADH Pyruvate from Glycolysis either enter the mitochondria (cellular respiration) or stays in cytosol ...
... Where does each reaction take place? (see your worksheet) o Glycolysis o Formation of acetyl CoA o Krebs cycle o Electron transport chain o Fermentation Glycolysis splits sugar to make ATP & NADH Pyruvate from Glycolysis either enter the mitochondria (cellular respiration) or stays in cytosol ...
First test material Study guide
... D. contains glycolipids that signify blood group antigens D. All the above Answer – E. all the above 2. What is the equation for bicarbonate buffer system within the blood? Discuss the effects of H+ on the ability for oxygen to bind to hemoglobin. Answer – Within tissues, the release of CO2 causes H ...
... D. contains glycolipids that signify blood group antigens D. All the above Answer – E. all the above 2. What is the equation for bicarbonate buffer system within the blood? Discuss the effects of H+ on the ability for oxygen to bind to hemoglobin. Answer – Within tissues, the release of CO2 causes H ...
Section 1 Workbook Unit 1 ANSWERS File
... that the functions are all involved in Protein Synthesis (building proteins) ...
... that the functions are all involved in Protein Synthesis (building proteins) ...
Ch. 9
... • The citric acid cycle, also called the Krebs cycle, takes place within the mitochondrial matrix • The cycle oxidizes organic fuel derived from pyruvate, generating one ATP, 3 NADH, and 1 FADH2 per turn ...
... • The citric acid cycle, also called the Krebs cycle, takes place within the mitochondrial matrix • The cycle oxidizes organic fuel derived from pyruvate, generating one ATP, 3 NADH, and 1 FADH2 per turn ...
Nitrogen Balance
... • Arginine and histidine contain five adjacent carbons and a sixth carbon attached through a nitrogen atom. • The catabolic conversion of these amino acids to glutamate is therefore slightly more complex than proline or glutamine. • Arginine is converted to the five-carbon skeleton of ornithine by a ...
... • Arginine and histidine contain five adjacent carbons and a sixth carbon attached through a nitrogen atom. • The catabolic conversion of these amino acids to glutamate is therefore slightly more complex than proline or glutamine. • Arginine is converted to the five-carbon skeleton of ornithine by a ...
E. coli - Department of Chemistry
... All of the first group are typically produced by simple chemical methodology. For example: Sorbitol by catalytic hydrogenation of glucose Levulinic acid by acid catalyzed dehydration of sugars Glucaric acid by oxidation of starch with nitric acid or hypochlorite ...
... All of the first group are typically produced by simple chemical methodology. For example: Sorbitol by catalytic hydrogenation of glucose Levulinic acid by acid catalyzed dehydration of sugars Glucaric acid by oxidation of starch with nitric acid or hypochlorite ...
Microbial Origins of Life and Energy Conversions
... • Microbes break down proteins into amino acids • A second set of microbes break amino acids down into ammonia ...
... • Microbes break down proteins into amino acids • A second set of microbes break amino acids down into ammonia ...
Biochemical Processes Check 3 (Solutions)
... Cells need energy to do cellular work. Processes that require energy are: cell division, synthesis of new parts and materials, muscular contraction, active transport and nervous conduction. 12. What is the difference between anabolic and catabolic reactions? Anabolic reactions are those involving th ...
... Cells need energy to do cellular work. Processes that require energy are: cell division, synthesis of new parts and materials, muscular contraction, active transport and nervous conduction. 12. What is the difference between anabolic and catabolic reactions? Anabolic reactions are those involving th ...
Energy systems & the continuum
... ATP is produced very slowly by the Aerobic System, it is very sluggish compared to the CP & Lactic Acid Systems. ...
... ATP is produced very slowly by the Aerobic System, it is very sluggish compared to the CP & Lactic Acid Systems. ...
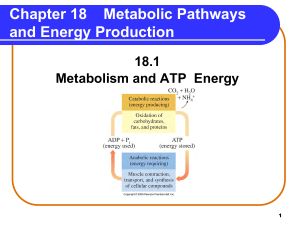
Metabolic Pathways and Energy Production
... Solution Match the following terms with the descriptions. 1) catabolic reactions 2) coenzymes 3) glycolysis 4) lactate A. 4 produced during anaerobic conditions B. 3 reaction series that converts glucose to pyruvate C. 1 metabolic reactions that break down large molecules to smaller molecules + ene ...
... Solution Match the following terms with the descriptions. 1) catabolic reactions 2) coenzymes 3) glycolysis 4) lactate A. 4 produced during anaerobic conditions B. 3 reaction series that converts glucose to pyruvate C. 1 metabolic reactions that break down large molecules to smaller molecules + ene ...
BIOLOGICAL OXIDATION
... It transfers electrons from ubiquinol to cytochrome c using cyt b and cyt c1 as coenzymes. Complex IV: Cytochrome oxidase (cytochrome-oxygen oxidoreductase) It transfers electrons from cytochrome c to oxygen. It needs cyt a and cyt a3 as coenzymes. ...
... It transfers electrons from ubiquinol to cytochrome c using cyt b and cyt c1 as coenzymes. Complex IV: Cytochrome oxidase (cytochrome-oxygen oxidoreductase) It transfers electrons from cytochrome c to oxygen. It needs cyt a and cyt a3 as coenzymes. ...
Notes - Organic Molecules of Life
... Sequence of nitrogenous bases codes for specific amino acids Amino acid sequence determines the ___________________ made in the cell and the cellular activity RNA - __________________ is its sugar backbone The base can be one of four: Adenine Guanine Cytosine ______________(replacesThymine) Only a s ...
... Sequence of nitrogenous bases codes for specific amino acids Amino acid sequence determines the ___________________ made in the cell and the cellular activity RNA - __________________ is its sugar backbone The base can be one of four: Adenine Guanine Cytosine ______________(replacesThymine) Only a s ...
Cellular respiration *vs
... •1. Glycolysis: this process that takes 1 glucose molecule, in the cell’s cytoplasm and breaks it down into 2 molecules of pyruvate which is used in the Kreb’s cycle (stage 2). This stage also releases 2 ATP and 2 water molecules. Also released are 2 •molecules of NADPH (Helps the body make sugar la ...
... •1. Glycolysis: this process that takes 1 glucose molecule, in the cell’s cytoplasm and breaks it down into 2 molecules of pyruvate which is used in the Kreb’s cycle (stage 2). This stage also releases 2 ATP and 2 water molecules. Also released are 2 •molecules of NADPH (Helps the body make sugar la ...
Exam 1
... 55. A common oxidizing agent used to couple chemical reactions in cells is A. riboflavin. *B. NADH. C. niacin. D. FAD. 56. A common reducing agent used to couple chemical reactions in cells is A. riboflavin. B. niacin. C. NADH. *D. FAD. 57. Metabolism is a term that refers to all of the reactions in ...
... 55. A common oxidizing agent used to couple chemical reactions in cells is A. riboflavin. *B. NADH. C. niacin. D. FAD. 56. A common reducing agent used to couple chemical reactions in cells is A. riboflavin. B. niacin. C. NADH. *D. FAD. 57. Metabolism is a term that refers to all of the reactions in ...
Urea Cycle Defect: A Case Study
... The presence of increased levels of ammonia affects the function of glutamate dehydrogenase, which converts the amino group of glutamate to ammonia. The increase in the plasma glutamine is due to the fact that the presence of glutamate is increased and an enzyme called glutaminase converts excess gl ...
... The presence of increased levels of ammonia affects the function of glutamate dehydrogenase, which converts the amino group of glutamate to ammonia. The increase in the plasma glutamine is due to the fact that the presence of glutamate is increased and an enzyme called glutaminase converts excess gl ...
Chapter 14 (Part 1)
... Outer Membrane – Freely permeable to small molecules and ions. Contains porins with 10,000 dalton limit Inner membrane – Protein rich (4:1 protein:lipid). Impermeable. Contains ETR, ATP synthase, transporters. Cristae – Highly folded inner membrane structure. Increase surface area. Matrix- “cytosol ...
... Outer Membrane – Freely permeable to small molecules and ions. Contains porins with 10,000 dalton limit Inner membrane – Protein rich (4:1 protein:lipid). Impermeable. Contains ETR, ATP synthase, transporters. Cristae – Highly folded inner membrane structure. Increase surface area. Matrix- “cytosol ...
Lecture_3_17012017
... 1. The quantity of enzyme present can be regulated at the level of gene transcription. ...
... 1. The quantity of enzyme present can be regulated at the level of gene transcription. ...
Biogeochemical Cycles PPT
... Wow. Talk about a word that describes everything on Earth. The cycles we discuss will all fall into the big group of biogeochemical cycles. Let's break it down. BIO: Biology. Life. Living things. These cycles all play a role in the lives of living things. The cycles might limit the organisms of Eart ...
... Wow. Talk about a word that describes everything on Earth. The cycles we discuss will all fall into the big group of biogeochemical cycles. Let's break it down. BIO: Biology. Life. Living things. These cycles all play a role in the lives of living things. The cycles might limit the organisms of Eart ...
Honors Bio – Key concepts for final
... How is energy harvested when electrons are trapped by NAD+ and then put through the electron transport chain? o Electrons are released along with hydrogen atoms as organic molecules (like glucose) are broken down ...
... How is energy harvested when electrons are trapped by NAD+ and then put through the electron transport chain? o Electrons are released along with hydrogen atoms as organic molecules (like glucose) are broken down ...
Citric acid cycle
The citric acid cycle – also known as the tricarboxylic acid (TCA) cycle or the Krebs cycle – is a series of chemical reactions used by all aerobic organisms to generate energy through the oxidation of acetate derived from carbohydrates, fats and proteins into carbon dioxide and chemical energy in the form of adenosine triphosphate (ATP). In addition, the cycle provides precursors of certain amino acids as well as the reducing agent NADH that is used in numerous other biochemical reactions. Its central importance to many biochemical pathways suggests that it was one of the earliest established components of cellular metabolism and may have originated abiogenically.The name of this metabolic pathway is derived from citric acid (a type of tricarboxylic acid) that is consumed and then regenerated by this sequence of reactions to complete the cycle. In addition, the cycle consumes acetate (in the form of acetyl-CoA) and water, reduces NAD+ to NADH, and produces carbon dioxide as a waste byproduct. The NADH generated by the TCA cycle is fed into the oxidative phosphorylation (electron transport) pathway. The net result of these two closely linked pathways is the oxidation of nutrients to produce usable chemical energy in the form of ATP.In eukaryotic cells, the citric acid cycle occurs in the matrix of the mitochondrion. In prokaryotic cells, such as bacteria which lack mitochondria, the TCA reaction sequence is performed in the cytosol with the proton gradient for ATP production being across the cell's surface (plasma membrane) rather than the inner membrane of the mitochondrion.