* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download PowerPoint 프레젠테이션
Biochemistry wikipedia , lookup
Biosynthesis wikipedia , lookup
Photosynthesis wikipedia , lookup
Western blot wikipedia , lookup
Microbial metabolism wikipedia , lookup
Mitochondrial replacement therapy wikipedia , lookup
Mitochondrion wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Metalloprotein wikipedia , lookup
Citric acid cycle wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Light-dependent reactions wikipedia , lookup
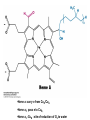
Ubiquinol (QH2) is also the Entry Point for Electrons From FADH2 of Flavoproteins • succinate dehydrogenase (integral membrane protein of inner mitochondrial membrane) which generates FADH2: • • part of succinate-Q reductase complex (Complex II) Electrons Flow From Ubiquinol to Cytochrome C Through Cytochrome Reductase • Cytochrome reductase: Ubiquinol-cytochrome c reductase, cytochrome, cytochrome bc1 complex. • cytochrome: electron-transferring protein that contains a heme prosthetic group. • The cytochrome b component of the reductase is in essence a recyling device that enables both electrons of QH2 to be effectively used. • structure of Q-cytochrome c oxidoreductase (cytochrome bc1): homodimer with 11 distinct polypeptide chains. •They are positioned to mediate e-transfer reactions between quinone and cyt c. Q cycle funnels e from a 2-e carrier to a 1-e carrier and pumps H • in the first half of cycle, 2e of bound QH2 are transferred, 1 to cyt C and 1 to bound Q to form semiquinone. The newly formed Q dissociates and enters Q pool. •In 2nd half of cycle, a second QH2 give up e, 1 to 2nd cytC, and 1 to reduce Q to QH2. 2nd e transfer results in uptake of 2H from matrix. •path of e transfer (red) Cytochrome c oxidase catalyzes the reduction of molecular oxygen to water • Four electrons are funneled into O2 to completely reduce it to H2O and concomitantly pump protons from the matrix to the cytosolic side of the inner mitochondrial membrane. • Heme a3 and Cu from the active center at which O2 is reduced to H2O. • Cytochrome c oxidase evolved to pump 4 additional H from the matrix to the cytoplasmic side of the membrane in the course of each reaction cycle for a total of 8 H removed from the matrix. •structure of cyt C oxidase: 13 polypeptide chain/ major prosthetic group: CuA/CuA, Heme a, Heme a3 -CuB • Heme a3 -CuB : site of reduction of O2 to water • CO(bb): carbonyl group of peptide backbone. •Heme a: carry e from CuA/CuA •Heme a3: pass e to CuB , •Heme a3 -CuB : site of reduction of O2 to water •Cytochrome c oxidase evolved to pump 4 additional H from the matrix to the cytoplasmic side of the membrane in the course of each reaction cycle for a total of 8 H removed from the matrix. • Danger lurks in the reduction of O2. • The transfer of a single e to O2 forms superoxide anion, whereas the transfer of 2 e yields peroxide. • The catalyst does not release partly reduced intermediates: cyt C oxidase (holding O2 tightly between Fe and Cu) •Peroxide bridge: O2 bound to heme a3 is reduced to peroxide by presence of CuB •proton transport by cyt C oxidase: • 4 H+ : taken from matrix to reduce 1 O2 to 2 H2O • 4 pumped H+: transported out of matrix and released on cytosolic side, double efficiency of free energy storage in form of proton gradient Toxic Derivatives of O2 Such as Superoxide Radical are Scavenged by Protective Enzymes •Superoxide dismutase: • oxidized form react with 1 superoxide ion to form O2 and generates reduced form of enzyme • reduced form react with 2nd superoxide and 2H+ to form hydrogen peroxide and regenerate enzyme The Conformation of Cytochrome c Has Remained Essentially Constant for More Than a Billion Years • The cytochrome c of any eukaryotic species reacts in vitro with the cytochrome oxidase of any other species tested thus far. • 26 of 104 residues have been invariant for more than one and a half billion years of evolution. • conservation of 3-D structure of cytochrome c: • 21 conserved amino acids and heme A proton gradient powers the synthesis of ATP • Mitochondrial ATPase: • ATP synthase (preferred name, emphasize its role in mitochondria) •chemiosmotic hypothesis: this proton-motive force drives the synthesis of ATP by the ATPase complex. • The primary energy-conserving event induced by electron transport is the generation of a proton-motive force across the inner mitochondrial membrane. •The respiratory chain and ATP synthase are biochemically separate systems, linked only by a proton-motive force. • ATP synthesized when reconstituted membrane vesicle containing bacteriorhodopsin (light-driven proton pump) and ATP synthase are illuminated. The orientation of ATP synthase is the reverse of that in the mitochondria. ATP synthase is composed of a protonconducting unit and a catalytic unit • Structure of ATP synthase (F0F1-ATPase): • F0 is the proton channel of the complex. • F1: 5 subunits • The gamma subunit breaks the symmetry of the alpha3beta3 hexamer: each of beta subunit is distinct by virtue of its interaction with a different face of gamma.