Structure of Proteins, Carbohydrates and Fats
... Fats are a sub-group of compounds known as lipids that are found in the body and have the general property of being hydrophobic (meaning they are insoluble in water). Fats are also known as triglycerides, molecules made from the combination of one molecule of glycerol with three fatty acids, as depi ...
... Fats are a sub-group of compounds known as lipids that are found in the body and have the general property of being hydrophobic (meaning they are insoluble in water). Fats are also known as triglycerides, molecules made from the combination of one molecule of glycerol with three fatty acids, as depi ...
A.P. Biology Summer Work: Worksheet
... A compound found mainly in living things is known as an organic compound. Organic compounds make up the cells and other structures of organisms and carry out life processes. Carbon is the main element in organic compounds, so carbon is essential to life on Earth. Without carbon, life as we know it c ...
... A compound found mainly in living things is known as an organic compound. Organic compounds make up the cells and other structures of organisms and carry out life processes. Carbon is the main element in organic compounds, so carbon is essential to life on Earth. Without carbon, life as we know it c ...
19 Dr. Nafez Abu Tarboosh Qusai Al Sharef
... carbonyl ketone group (on C 2) so the bonds around this carbon will be weaken (between c1 and c2) and C1 will leave as a carboxylic group and this is why we call it decarboxylation reaction. Thiamine is rapidly converted to its active form thiamine pyrophosphate (TPP) in the brain and liver. ...
... carbonyl ketone group (on C 2) so the bonds around this carbon will be weaken (between c1 and c2) and C1 will leave as a carboxylic group and this is why we call it decarboxylation reaction. Thiamine is rapidly converted to its active form thiamine pyrophosphate (TPP) in the brain and liver. ...
The Lactic Acid System
... Many think that lactic acid or lactate cause muscle stiffness and limit performance in running events. Neither is correct. Perhaps the most widely believed myth is that an accumulation of lactic acid or lactic acid crystals or lactate is the cause of the stiffness felt after a marathon or long run. ...
... Many think that lactic acid or lactate cause muscle stiffness and limit performance in running events. Neither is correct. Perhaps the most widely believed myth is that an accumulation of lactic acid or lactic acid crystals or lactate is the cause of the stiffness felt after a marathon or long run. ...
The Dna code - Winston Knoll Collegiate
... DNA stores information to build proteins in sequences of nucleotides - DNA nucleotides contain one of 4 nitrogen bases A T C G - there are 20 different amino acids used to build protein ...
... DNA stores information to build proteins in sequences of nucleotides - DNA nucleotides contain one of 4 nitrogen bases A T C G - there are 20 different amino acids used to build protein ...
Lactic Acid : Brief History
... cycle (TCA cycle), or the Krebs cycle, is a series of chemical reactions used by all aerobic organisms to generate energy through the oxidation ...
... cycle (TCA cycle), or the Krebs cycle, is a series of chemical reactions used by all aerobic organisms to generate energy through the oxidation ...
HEMOGLOBIN
... due to: (a) incompatible blood transfusion (b) hemolytic anemia 2) HEPATIC JAUNDICE: can be due to: A) Decreased glucouronyl transferase as in physiologic jaundice of newborn. There is increase in unconjugated lipid soluble bilirubin which can cross the blood brain barrier and produce encephalopathy ...
... due to: (a) incompatible blood transfusion (b) hemolytic anemia 2) HEPATIC JAUNDICE: can be due to: A) Decreased glucouronyl transferase as in physiologic jaundice of newborn. There is increase in unconjugated lipid soluble bilirubin which can cross the blood brain barrier and produce encephalopathy ...
CO 2
... C4 plants PHYSICALLY separate carbon fixation from Calvin cycle different cells to fix carbon vs. where Calvin cycle occurs store carbon in 4C compounds different enzyme to capture CO2 (fix carbon) PEP carboxylase ...
... C4 plants PHYSICALLY separate carbon fixation from Calvin cycle different cells to fix carbon vs. where Calvin cycle occurs store carbon in 4C compounds different enzyme to capture CO2 (fix carbon) PEP carboxylase ...
Lipid Synthesis
... b. Stored and broken down for specific cell needs c. Why do cells go through process of making fatty acids? d. In developed nations, it’s not so critical, but it does occur in two different ways i. De novo synthesis – like making from scratch ii. Carbon lengthening of Palmitic acid and the formation ...
... b. Stored and broken down for specific cell needs c. Why do cells go through process of making fatty acids? d. In developed nations, it’s not so critical, but it does occur in two different ways i. De novo synthesis – like making from scratch ii. Carbon lengthening of Palmitic acid and the formation ...
Chem 356 Structure and Function in Biochemistry
... glucose by microorganisms. The glucose is then broken down to pyruvate via glycolysis. Because the process is carried out in the absence of oxygen (i.e., it is fermentation), pyuvate is reduced to lactic acid and ethanol by the microorganisms. If oxygen were present, pyruvate would be oxidized to ac ...
... glucose by microorganisms. The glucose is then broken down to pyruvate via glycolysis. Because the process is carried out in the absence of oxygen (i.e., it is fermentation), pyuvate is reduced to lactic acid and ethanol by the microorganisms. If oxygen were present, pyruvate would be oxidized to ac ...
Week 03 Lecture notes
... • The “opposite” of photosynthesis; high-energy electrons are harvested by breaking down sugars • Electrons pass through ETS in mitochondria Again, the energy from the electron is used to power proton pumps and generate a proton gradient across the membrane Flow of protons down the gradient thro ...
... • The “opposite” of photosynthesis; high-energy electrons are harvested by breaking down sugars • Electrons pass through ETS in mitochondria Again, the energy from the electron is used to power proton pumps and generate a proton gradient across the membrane Flow of protons down the gradient thro ...
http://www - bu people
... 6. Draw the ionized and nonionized forms of acidic and basic residues and note the approximate pH range in which these forms exist. 6. In nonionized histidine, the imidazole ring can exist as two tautomers, with the hydrogen atom on either nitrogen atom. The ring is readily protonated, with a pKa va ...
... 6. Draw the ionized and nonionized forms of acidic and basic residues and note the approximate pH range in which these forms exist. 6. In nonionized histidine, the imidazole ring can exist as two tautomers, with the hydrogen atom on either nitrogen atom. The ring is readily protonated, with a pKa va ...
Problem-Set Solutions
... phosphoenolpyruvate, can also act as an intermediate in the first step of the citric acid cycle; oxaloacetate combines with acetyl CoA, which can go directly into the citric acid cycle. 24.70 GTP and ATP 24.71 Lactate formed by muscle activity diffuses into the blood and is carried to the liver wher ...
... phosphoenolpyruvate, can also act as an intermediate in the first step of the citric acid cycle; oxaloacetate combines with acetyl CoA, which can go directly into the citric acid cycle. 24.70 GTP and ATP 24.71 Lactate formed by muscle activity diffuses into the blood and is carried to the liver wher ...
Differential effects of heptanoate and hexanoate on myocardial citric
... an infusion of either 1) saline (Con); 2) sodium heptanoate (Hep) (50 mM; Sigma-Aldrich); or 3) sodium hexanoate (Hex) (50 mM; SigmaAldrich) in NaCl at 308 mosmol/kgH2O into the perfusion circuit at 8 l/min for every milliliter of blood flow in the LAD perfusion circuit. This infusion rate was aime ...
... an infusion of either 1) saline (Con); 2) sodium heptanoate (Hep) (50 mM; Sigma-Aldrich); or 3) sodium hexanoate (Hex) (50 mM; SigmaAldrich) in NaCl at 308 mosmol/kgH2O into the perfusion circuit at 8 l/min for every milliliter of blood flow in the LAD perfusion circuit. This infusion rate was aime ...
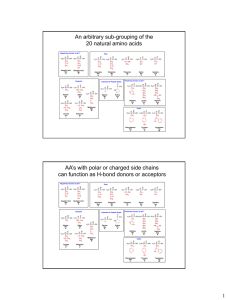
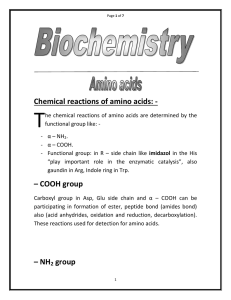
Chemical reactions of amino acids:
... This reaction favors peptide bond hydrolysis, so to synthesis peptide bond, the COOH group must be first activated. Chemically this done by conversion to an acid chloride. Biologically done by condensation with ATP forming an amino acyladenlate. There are different reactions for detections ami ...
... This reaction favors peptide bond hydrolysis, so to synthesis peptide bond, the COOH group must be first activated. Chemically this done by conversion to an acid chloride. Biologically done by condensation with ATP forming an amino acyladenlate. There are different reactions for detections ami ...
Metabolic production and renal disposal of hydrogen ions
... liver is thus estimated at about 138 mEq per day. glucose or carbon dioxide, in proportions which vary from In contrast to the above, the metabolism of anions to neutral species to species. The two possible pathways are in fact end-products will remove hydrogen ions. Again, following equivalent in t ...
... liver is thus estimated at about 138 mEq per day. glucose or carbon dioxide, in proportions which vary from In contrast to the above, the metabolism of anions to neutral species to species. The two possible pathways are in fact end-products will remove hydrogen ions. Again, following equivalent in t ...
plasma membrane - Cengage Learning
... ATP transfers energy in many different chemical reactions; almost all metabolic pathways directly or indirectly run on energy supplied by ATP. ATP can donate a phosphate group (phosphorylation) to another molecule, which then becomes primed and energized for specific reactions. ...
... ATP transfers energy in many different chemical reactions; almost all metabolic pathways directly or indirectly run on energy supplied by ATP. ATP can donate a phosphate group (phosphorylation) to another molecule, which then becomes primed and energized for specific reactions. ...
Bacteria - Eubacteria
... C6H12O6 C3H3O3- C2H5OH + CO2 C6H12O6 C3H3O3- H3CCHOHCOONotice how fermentation can produce gas or acids… These are just a few of the fermentive possibilities! ...
... C6H12O6 C3H3O3- C2H5OH + CO2 C6H12O6 C3H3O3- H3CCHOHCOONotice how fermentation can produce gas or acids… These are just a few of the fermentive possibilities! ...
Organic Chemistry Answer Key
... A pH change can cause the enzyme to change its shape. A pH change can remove energy necessary to activate an enzyme. A pH change can add new molecules to the structure of the enzyme. A pH change can cause an enzyme to react with a different substrate. ...
... A pH change can cause the enzyme to change its shape. A pH change can remove energy necessary to activate an enzyme. A pH change can add new molecules to the structure of the enzyme. A pH change can cause an enzyme to react with a different substrate. ...
File
... provided. After each molecule is made, the group will come together and follow the instructions and answer the questions regarding carbohydrates. Monosaccharide’s (single molecules of sugar) A single molecule of sugar is called a monosaccharide. The prefix “Mono” means one. However, the one molecule ...
... provided. After each molecule is made, the group will come together and follow the instructions and answer the questions regarding carbohydrates. Monosaccharide’s (single molecules of sugar) A single molecule of sugar is called a monosaccharide. The prefix “Mono” means one. However, the one molecule ...
Can you describe the various methods of cell membrane transport?
... lymphocyte cell—a number of mitochondria are visible. ...
... lymphocyte cell—a number of mitochondria are visible. ...
Sample lab - eScience Labs
... words, they break down big molecules into smaller pieces. Free energy is released when the molecules are broken down, which can be sequentially used in each proceeding step. Through a series of oxidation reactions, the cell converts carbohydrates into carbon dioxide and water. It also transforms ade ...
... words, they break down big molecules into smaller pieces. Free energy is released when the molecules are broken down, which can be sequentially used in each proceeding step. Through a series of oxidation reactions, the cell converts carbohydrates into carbon dioxide and water. It also transforms ade ...
Citric acid cycle
The citric acid cycle – also known as the tricarboxylic acid (TCA) cycle or the Krebs cycle – is a series of chemical reactions used by all aerobic organisms to generate energy through the oxidation of acetate derived from carbohydrates, fats and proteins into carbon dioxide and chemical energy in the form of adenosine triphosphate (ATP). In addition, the cycle provides precursors of certain amino acids as well as the reducing agent NADH that is used in numerous other biochemical reactions. Its central importance to many biochemical pathways suggests that it was one of the earliest established components of cellular metabolism and may have originated abiogenically.The name of this metabolic pathway is derived from citric acid (a type of tricarboxylic acid) that is consumed and then regenerated by this sequence of reactions to complete the cycle. In addition, the cycle consumes acetate (in the form of acetyl-CoA) and water, reduces NAD+ to NADH, and produces carbon dioxide as a waste byproduct. The NADH generated by the TCA cycle is fed into the oxidative phosphorylation (electron transport) pathway. The net result of these two closely linked pathways is the oxidation of nutrients to produce usable chemical energy in the form of ATP.In eukaryotic cells, the citric acid cycle occurs in the matrix of the mitochondrion. In prokaryotic cells, such as bacteria which lack mitochondria, the TCA reaction sequence is performed in the cytosol with the proton gradient for ATP production being across the cell's surface (plasma membrane) rather than the inner membrane of the mitochondrion.