unit 1: introduction to biology
... 2 mechanisms are used by cells to synthesize the cellular fuel ATP 1. by chemio-osmotic phosphorylation or short: chemio-osmosis also called ‘Mitchell or chemio-osmotic theory’, named after the British biochemist Peter Mitchell who first described this mechanism in the 1960s it is called osmosis ...
... 2 mechanisms are used by cells to synthesize the cellular fuel ATP 1. by chemio-osmotic phosphorylation or short: chemio-osmosis also called ‘Mitchell or chemio-osmotic theory’, named after the British biochemist Peter Mitchell who first described this mechanism in the 1960s it is called osmosis ...
ANSWERS - Unit 1 Review File
... amino groups, bases release glycerol d) acids release hydroxide ions, bases release hydrogen ions. 31. The process that joins amino acids together to make enzymes is: a)oxidation b) hydrolysis c)denaturation d) dehydration synthesis 32. Which of the following is an amino (amine) group? a)NH2 b)OH-1 ...
... amino groups, bases release glycerol d) acids release hydroxide ions, bases release hydrogen ions. 31. The process that joins amino acids together to make enzymes is: a)oxidation b) hydrolysis c)denaturation d) dehydration synthesis 32. Which of the following is an amino (amine) group? a)NH2 b)OH-1 ...
THE METABOLISM OF KETONE BODIES
... production that exceeds the ability of peripheral tissues to oxidize them. • Ketone bodies are relatively strong acids (pKa around 3.5), and their increase lowers the pH of the blood. This acidification of the blood is dangerous chiefly because it impairs the ability of hemoglobin to bind oxygen. ...
... production that exceeds the ability of peripheral tissues to oxidize them. • Ketone bodies are relatively strong acids (pKa around 3.5), and their increase lowers the pH of the blood. This acidification of the blood is dangerous chiefly because it impairs the ability of hemoglobin to bind oxygen. ...
Exam #2 Review
... pathways must be dynamic and coordinated so that cells can respond to changes in environment. Each reaction is catalyzed by a specific enzyme. Every enzyme-catalyzed reaction represents a potential point of regulation (inhibition or activation). In catabolic pathways the starting compound (an energy ...
... pathways must be dynamic and coordinated so that cells can respond to changes in environment. Each reaction is catalyzed by a specific enzyme. Every enzyme-catalyzed reaction represents a potential point of regulation (inhibition or activation). In catabolic pathways the starting compound (an energy ...
Notes ch 2 the nature of matter
... Sea water Human blood Pure water Milk Normal rainfall Acid rain Tomato juice Lemon juice Stomach acid ...
... Sea water Human blood Pure water Milk Normal rainfall Acid rain Tomato juice Lemon juice Stomach acid ...
Biochemistry Unit Homework (Chapters 5 and 8)
... 2. Contrast and compare cofactors versus coenzymes. 3. Contrast and compare competitive inhibitors with noncompetitive inhibitors. Which can be overcome by the addition of more substrate? 4. Discuss which level of protein structure is most directly responsible for the specificity of an enzyme. 5. In ...
... 2. Contrast and compare cofactors versus coenzymes. 3. Contrast and compare competitive inhibitors with noncompetitive inhibitors. Which can be overcome by the addition of more substrate? 4. Discuss which level of protein structure is most directly responsible for the specificity of an enzyme. 5. In ...
Atomic Structure (Bohr or Planetary Model)
... – glucose, maltose, amylose, fructose, sucrose • The monomer of carbohydrates is the monosaccharide (one sugar) of which there are a number of types – glucose is the most biologically important • Carbon:Hydrogen:Oxygen in a 1:2:1 atomic ratio – glucose = C6H12O6 • Because they contain oxygen, they a ...
... – glucose, maltose, amylose, fructose, sucrose • The monomer of carbohydrates is the monosaccharide (one sugar) of which there are a number of types – glucose is the most biologically important • Carbon:Hydrogen:Oxygen in a 1:2:1 atomic ratio – glucose = C6H12O6 • Because they contain oxygen, they a ...
Slide 1
... There is an ideal sequence of redox reactions driven by e- rich organic matter that is based on the energy available for the microbes that mediate the reactions. ...
... There is an ideal sequence of redox reactions driven by e- rich organic matter that is based on the energy available for the microbes that mediate the reactions. ...
as Powerpoint presentation
... Peter Mitchell, 1961. The Chemiosmotic Hypothesis. Mitchell won the Nobel Prize for this in 1978 Mitchell proposed an indirect interaction between oxidizing and phosphorylating enzymes. The flow of electrons through the enzymes of the respiratory or photosynthetic electron-transfer chains drives pos ...
... Peter Mitchell, 1961. The Chemiosmotic Hypothesis. Mitchell won the Nobel Prize for this in 1978 Mitchell proposed an indirect interaction between oxidizing and phosphorylating enzymes. The flow of electrons through the enzymes of the respiratory or photosynthetic electron-transfer chains drives pos ...
Chapter 2 The Chemistry of Life
... I can – distinguish between acids and bases I can – perform multiple pH tests to draw ...
... I can – distinguish between acids and bases I can – perform multiple pH tests to draw ...
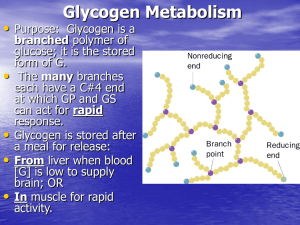
Glycogen Metabolism - http://www.utm.edu
... Carbamoyl Phosphate Synthetase I Regulation CPSI is activated by N-acetylglutamate NAG MR: NAG is produced from ACoA and glu: ACoA + glu NAG. A high [ACoA] and/or a high [glu] increases the rate of NAG production and the [NAG], so the [NAG] indicates the levels of AcoA and glu. MR, ML for ACoA: wh ...
... Carbamoyl Phosphate Synthetase I Regulation CPSI is activated by N-acetylglutamate NAG MR: NAG is produced from ACoA and glu: ACoA + glu NAG. A high [ACoA] and/or a high [glu] increases the rate of NAG production and the [NAG], so the [NAG] indicates the levels of AcoA and glu. MR, ML for ACoA: wh ...
File
... - Can exist in a chain or a ring • Everything that happens in our body is due to changes in levels of molecules: 1) make new molecules 2) maintain existing molecules 3) dispose of dead molecules ...
... - Can exist in a chain or a ring • Everything that happens in our body is due to changes in levels of molecules: 1) make new molecules 2) maintain existing molecules 3) dispose of dead molecules ...
Block 1 Unit 2 Objectives Bone Tissue Objectives List and describe
... 3. Cartilage develops when mesenchymal cells condense to form procartilage. The procartilage will secrete a cartilage matrix. 4. Integrate the characteristics of individual components of CT to the structure and function of Cartilage 5. Morphology fits function for the three types of cartilage. Hyali ...
... 3. Cartilage develops when mesenchymal cells condense to form procartilage. The procartilage will secrete a cartilage matrix. 4. Integrate the characteristics of individual components of CT to the structure and function of Cartilage 5. Morphology fits function for the three types of cartilage. Hyali ...
8.1 Energy and Life
... to higher energy levels. These high-energy electrons are used in photosynthesis. Electron carriers are used to transport the electrons from chlorophyll to other molecules during photosynthesis. NADP+ is a compound that can accept and hold 2 high-energy electrons and 1 hydrogen ion. This process conv ...
... to higher energy levels. These high-energy electrons are used in photosynthesis. Electron carriers are used to transport the electrons from chlorophyll to other molecules during photosynthesis. NADP+ is a compound that can accept and hold 2 high-energy electrons and 1 hydrogen ion. This process conv ...
Bioconversion - Portal UniMAP
... 3. Factors influence-Oxygen supply and the concentration gradients of ethanol and acetate. ...
... 3. Factors influence-Oxygen supply and the concentration gradients of ethanol and acetate. ...
Studies on the Fate of Isotopically Labeled
... genates. Any failure of pyruvate to undergo oxida tion could conceivably result in its competing for electrons with other electron acceptors, thus lead ing to lactic acid formation. I will defer discussion of the third possibility until later. The question with which we concerned our selves in the p ...
... genates. Any failure of pyruvate to undergo oxida tion could conceivably result in its competing for electrons with other electron acceptors, thus lead ing to lactic acid formation. I will defer discussion of the third possibility until later. The question with which we concerned our selves in the p ...
Exam 2 question possibility for 2008
... (both 3H and BUdR in the same sample) (neither BUdR nor 3H). Circle all correct possibilities. B-3. You isolate DNA of each density, denature it, and measure the Tm. BUdR is in the enol form more often than T is. Assume that in the ds DNA made in expt. 2, BUdR is found only where T belongs, so it is ...
... (both 3H and BUdR in the same sample) (neither BUdR nor 3H). Circle all correct possibilities. B-3. You isolate DNA of each density, denature it, and measure the Tm. BUdR is in the enol form more often than T is. Assume that in the ds DNA made in expt. 2, BUdR is found only where T belongs, so it is ...
ENERGY SYSTEMS
... Energy provided by food is measured in kilojoules (kJ) When food is digested, it breaks down to sugars, amino acids and fatty acids – substances that become available as a usable form of energy From these, ATP or adenosine triphosphate is produced and represents the most important substance in energ ...
... Energy provided by food is measured in kilojoules (kJ) When food is digested, it breaks down to sugars, amino acids and fatty acids – substances that become available as a usable form of energy From these, ATP or adenosine triphosphate is produced and represents the most important substance in energ ...
Citric acid cycle
The citric acid cycle – also known as the tricarboxylic acid (TCA) cycle or the Krebs cycle – is a series of chemical reactions used by all aerobic organisms to generate energy through the oxidation of acetate derived from carbohydrates, fats and proteins into carbon dioxide and chemical energy in the form of adenosine triphosphate (ATP). In addition, the cycle provides precursors of certain amino acids as well as the reducing agent NADH that is used in numerous other biochemical reactions. Its central importance to many biochemical pathways suggests that it was one of the earliest established components of cellular metabolism and may have originated abiogenically.The name of this metabolic pathway is derived from citric acid (a type of tricarboxylic acid) that is consumed and then regenerated by this sequence of reactions to complete the cycle. In addition, the cycle consumes acetate (in the form of acetyl-CoA) and water, reduces NAD+ to NADH, and produces carbon dioxide as a waste byproduct. The NADH generated by the TCA cycle is fed into the oxidative phosphorylation (electron transport) pathway. The net result of these two closely linked pathways is the oxidation of nutrients to produce usable chemical energy in the form of ATP.In eukaryotic cells, the citric acid cycle occurs in the matrix of the mitochondrion. In prokaryotic cells, such as bacteria which lack mitochondria, the TCA reaction sequence is performed in the cytosol with the proton gradient for ATP production being across the cell's surface (plasma membrane) rather than the inner membrane of the mitochondrion.