File - Wk 1-2
... products of the cycle and the role of the cycle in providing reducing equivalents for the electron transport chain. The citric acid cycle (Krebs cycle) occurs in the mitacholdria of the cell and occurs in the presence of oxygen (aerobic pathway). Pyruvic acid from glycolysis first needs to be conver ...
... products of the cycle and the role of the cycle in providing reducing equivalents for the electron transport chain. The citric acid cycle (Krebs cycle) occurs in the mitacholdria of the cell and occurs in the presence of oxygen (aerobic pathway). Pyruvic acid from glycolysis first needs to be conver ...
How to Assess Patient Biochemical and Nutritional Metametrix Clinical Laboratory
... Glutathione is the main intercellular antioxidant of the liver. Evaluation of the use of glutathione is of great utility when judging liver function. A number of urine markers can help make this evaluation. A low sulfate reveals a need to replenish sulfur containing amino acids. Glutathione administ ...
... Glutathione is the main intercellular antioxidant of the liver. Evaluation of the use of glutathione is of great utility when judging liver function. A number of urine markers can help make this evaluation. A low sulfate reveals a need to replenish sulfur containing amino acids. Glutathione administ ...
Nerve activates contraction
... from body cells to the liver for breakdown; are increased by exercise, and limited coffee, smoking, and saturated fats/trans fats Healthy Ratios in the Blood ...
... from body cells to the liver for breakdown; are increased by exercise, and limited coffee, smoking, and saturated fats/trans fats Healthy Ratios in the Blood ...
Lecture 28 - Citrate Cycle
... • The primary function of the citrate cycle is to convert energy available from the oxidization acetyl-CoA into 3 moles of NADH, 1 mole of FADH2 and 1 mole of GTP during each turn of the cycle. • The citrate cycle is a "metabolic engine" in which all eight of the cycle intermediates are continually ...
... • The primary function of the citrate cycle is to convert energy available from the oxidization acetyl-CoA into 3 moles of NADH, 1 mole of FADH2 and 1 mole of GTP during each turn of the cycle. • The citrate cycle is a "metabolic engine" in which all eight of the cycle intermediates are continually ...
Unit Title:
... ENRG1. Identify the reactants, products, and basic purposes of photosynthesis and cellular respiration. Explain the interrelated nature of photosynthesis and cellular respiration in the cells of photosynthetic organisms. (2.4) ENRG2. Explain the important role that ATP serves in metabolism. (2.5) EN ...
... ENRG1. Identify the reactants, products, and basic purposes of photosynthesis and cellular respiration. Explain the interrelated nature of photosynthesis and cellular respiration in the cells of photosynthetic organisms. (2.4) ENRG2. Explain the important role that ATP serves in metabolism. (2.5) EN ...
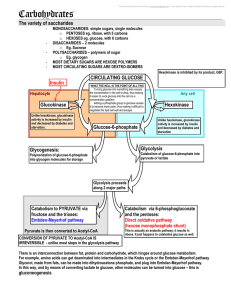
Reaction of glycolysis
... tissues, such as actively metabolizing muscle. NAD+ is recycled in the process • In some organisms, pyruvate is converted to ethanol in a process requiring thiamine pyrophosphate as a coenzyme ...
... tissues, such as actively metabolizing muscle. NAD+ is recycled in the process • In some organisms, pyruvate is converted to ethanol in a process requiring thiamine pyrophosphate as a coenzyme ...
5 carbohydrates and the Krebs Cycle
... the whole point is to convert Acetyl-CoA to CO2 and hydrogen. Acetyl-CoA is the major entry point, but amino acids when deaminated can enter at various points along the cycle This cycle requires O2 and does not function under anaerobic conditions ...
... the whole point is to convert Acetyl-CoA to CO2 and hydrogen. Acetyl-CoA is the major entry point, but amino acids when deaminated can enter at various points along the cycle This cycle requires O2 and does not function under anaerobic conditions ...
1A. Growing Plants - The Royal Society of Chemistry
... Nitrogen, the third main plant food is a gas and it is unreactive. So how are these important elements made available to plants by the soil? The only way is for them to be joined with other chemical elements to form chemical compounds. For a compound to be used by the plant it must be soluble in wat ...
... Nitrogen, the third main plant food is a gas and it is unreactive. So how are these important elements made available to plants by the soil? The only way is for them to be joined with other chemical elements to form chemical compounds. For a compound to be used by the plant it must be soluble in wat ...
respiration revision quiz
... In the reactions of respiration, coenzymes become …………………. as substrates become …………………….. . These are necessary because the reactions are catalysed by inefficient dehydrogenase ………………… . Hydrogen ATO ...
... In the reactions of respiration, coenzymes become …………………. as substrates become …………………….. . These are necessary because the reactions are catalysed by inefficient dehydrogenase ………………… . Hydrogen ATO ...
Chapter 25
... Figure 25.2 The novel prosthetic groups of nitrate reductase and nitrite reductase. (a) The molybdenum cofactor of nitrate reductase. The molybdenum-free version of this compound is a pterin derivative called molybdopterin. (b) Siroheme, a uroporphyrin derivative, is a member of the isobacteriochlor ...
... Figure 25.2 The novel prosthetic groups of nitrate reductase and nitrite reductase. (a) The molybdenum cofactor of nitrate reductase. The molybdenum-free version of this compound is a pterin derivative called molybdopterin. (b) Siroheme, a uroporphyrin derivative, is a member of the isobacteriochlor ...
Max Stieve Lesson Plans
... In a food web, a change in one population will only affect another population if the two populations are directly related as a predator and prey. Organisms higher in the food web eat everything that is lower in the food web. Varying the population size of species will only affect the others that are ...
... In a food web, a change in one population will only affect another population if the two populations are directly related as a predator and prey. Organisms higher in the food web eat everything that is lower in the food web. Varying the population size of species will only affect the others that are ...
100 Pectin is a complex polysaccharide consisting mainly of
... Pectinases are a group of enzymes that contribute to the breakdown of pectin which are structural polysaccharide found in primary cell wall and middle lamella of fruits and vegetables. Pectolysis is one of the most important processes for plant, as it plays a role in cell elongation and growth as we ...
... Pectinases are a group of enzymes that contribute to the breakdown of pectin which are structural polysaccharide found in primary cell wall and middle lamella of fruits and vegetables. Pectolysis is one of the most important processes for plant, as it plays a role in cell elongation and growth as we ...
L9 PS Variations Fa08
... • Uses cyclic electron flow to generate extra ATP • Occurs in bundle-sheath cells – Thylakoids only have PS1 (and cytochrome ...
... • Uses cyclic electron flow to generate extra ATP • Occurs in bundle-sheath cells – Thylakoids only have PS1 (and cytochrome ...
BCHM 463 Supplemental Problems for Friday, April 2, 2004 1. Write
... 2. During glycolysis, how many ADP molecules are converted to ATP. Explain this answer with regard to your answer to #1. 4 ADP molecules are converted into ATP. There is a net gain of only 2 ATP molecules because 2 are consumed during the first stage of glycolysis. 3. What are the three metabolicall ...
... 2. During glycolysis, how many ADP molecules are converted to ATP. Explain this answer with regard to your answer to #1. 4 ADP molecules are converted into ATP. There is a net gain of only 2 ATP molecules because 2 are consumed during the first stage of glycolysis. 3. What are the three metabolicall ...
VEN124 Section III
... containing amino acids • From reaction of reduced sulfur intermediates with other cellular metabolites? • Formed chemically due to reduced conditions? ...
... containing amino acids • From reaction of reduced sulfur intermediates with other cellular metabolites? • Formed chemically due to reduced conditions? ...
PDF
... et al., 2007), which is similar to the 3HOP bi-cycle and uses the same carbon assimilation enzymes (e.g., acetyl-CoA carboxylase and propionyl-CoA carboxylase), the dicarboxylate/4HOB cycle (Huber et al., 2008), and the reductive acetyl-CoA pathway (Ljungdahl, 1986; Wood, 1991) have not yet been rep ...
... et al., 2007), which is similar to the 3HOP bi-cycle and uses the same carbon assimilation enzymes (e.g., acetyl-CoA carboxylase and propionyl-CoA carboxylase), the dicarboxylate/4HOB cycle (Huber et al., 2008), and the reductive acetyl-CoA pathway (Ljungdahl, 1986; Wood, 1991) have not yet been rep ...
PDF
... et al., 2007), which is similar to the 3HOP bi-cycle and uses the same carbon assimilation enzymes (e.g., acetyl-CoA carboxylase and propionyl-CoA carboxylase), the dicarboxylate/4HOB cycle (Huber et al., 2008), and the reductive acetyl-CoA pathway (Ljungdahl, 1986; Wood, 1991) have not yet been rep ...
... et al., 2007), which is similar to the 3HOP bi-cycle and uses the same carbon assimilation enzymes (e.g., acetyl-CoA carboxylase and propionyl-CoA carboxylase), the dicarboxylate/4HOB cycle (Huber et al., 2008), and the reductive acetyl-CoA pathway (Ljungdahl, 1986; Wood, 1991) have not yet been rep ...
ATP Synthesis
... In the so-called “binding change” mechanism, each of the three αβ catalytic protomers of the α3β3 subunits of F1 component is envisioned to adopt three distinct conformations designated O, L and T that are in equilibrium exchange with each other: O catalytically-inactive / low affinity for ligands ...
... In the so-called “binding change” mechanism, each of the three αβ catalytic protomers of the α3β3 subunits of F1 component is envisioned to adopt three distinct conformations designated O, L and T that are in equilibrium exchange with each other: O catalytically-inactive / low affinity for ligands ...
C - 鄭智美的Homepage
... NAD+ as an electron shuttle • Electrons from organic compounds – Are usually first transferred to NAD+, a coenzyme 2 e– + 2 H+ ...
... NAD+ as an electron shuttle • Electrons from organic compounds – Are usually first transferred to NAD+, a coenzyme 2 e– + 2 H+ ...
Molecular Biology of the Cell
... lactate does not directly cause acidosis, nor is it responsible for delayed onset muscle soreness. This is because lactate itself is not capable of releasing a proton. The acidosis that is associated with increases in lactate concentration during heavy exercise arises from a separate reaction. When ...
... lactate does not directly cause acidosis, nor is it responsible for delayed onset muscle soreness. This is because lactate itself is not capable of releasing a proton. The acidosis that is associated with increases in lactate concentration during heavy exercise arises from a separate reaction. When ...
Microbial metabolism
Microbial metabolism is the means by which a microbe obtains the energy and nutrients (e.g. carbon) it needs to live and reproduce. Microbes use many different types of metabolic strategies and species can often be differentiated from each other based on metabolic characteristics. The specific metabolic properties of a microbe are the major factors in determining that microbe’s ecological niche, and often allow for that microbe to be useful in industrial processes or responsible for biogeochemical cycles.== Types of microbial metabolism ==All microbial metabolisms can be arranged according to three principles:1. How the organism obtains carbon for synthesising cell mass: autotrophic – carbon is obtained from carbon dioxide (CO2) heterotrophic – carbon is obtained from organic compounds mixotrophic – carbon is obtained from both organic compounds and by fixing carbon dioxide2. How the organism obtains reducing equivalents used either in energy conservation or in biosynthetic reactions: lithotrophic – reducing equivalents are obtained from inorganic compounds organotrophic – reducing equivalents are obtained from organic compounds3. How the organism obtains energy for living and growing: chemotrophic – energy is obtained from external chemical compounds phototrophic – energy is obtained from lightIn practice, these terms are almost freely combined. Typical examples are as follows: chemolithoautotrophs obtain energy from the oxidation of inorganic compounds and carbon from the fixation of carbon dioxide. Examples: Nitrifying bacteria, Sulfur-oxidizing bacteria, Iron-oxidizing bacteria, Knallgas-bacteria photolithoautotrophs obtain energy from light and carbon from the fixation of carbon dioxide, using reducing equivalents from inorganic compounds. Examples: Cyanobacteria (water (H2O) as reducing equivalent donor), Chlorobiaceae, Chromatiaceae (hydrogen sulfide (H2S) as reducing equivalent donor), Chloroflexus (hydrogen (H2) as reducing equivalent donor) chemolithoheterotrophs obtain energy from the oxidation of inorganic compounds, but cannot fix carbon dioxide (CO2). Examples: some Thiobacilus, some Beggiatoa, some Nitrobacter spp., Wolinella (with H2 as reducing equivalent donor), some Knallgas-bacteria, some sulfate-reducing bacteria chemoorganoheterotrophs obtain energy, carbon, and reducing equivalents for biosynthetic reactions from organic compounds. Examples: most bacteria, e. g. Escherichia coli, Bacillus spp., Actinobacteria photoorganoheterotrophs obtain energy from light, carbon and reducing equivalents for biosynthetic reactions from organic compounds. Some species are strictly heterotrophic, many others can also fix carbon dioxide and are mixotrophic. Examples: Rhodobacter, Rhodopseudomonas, Rhodospirillum, Rhodomicrobium, Rhodocyclus, Heliobacterium, Chloroflexus (alternatively to photolithoautotrophy with hydrogen)