Oxidative Phosphorylation in Homogenates of
... The data in the present study show that a valid assay for the oxidizing enzymes of the Krebs cycle can be obtained only during the period in which the phosphate balance is being maintained, and that in homogenates in which no phase II occurs the period of valid oxygen uptake rate may be very brief a ...
... The data in the present study show that a valid assay for the oxidizing enzymes of the Krebs cycle can be obtained only during the period in which the phosphate balance is being maintained, and that in homogenates in which no phase II occurs the period of valid oxygen uptake rate may be very brief a ...
Phosphoketolase pathway dominates in
... both the Embden-Meyerhof pathway (EMP) and phosphoketolase pathway (PKP) when glucose or sucrose is converted into the three-carbon intermediate stage of glycolysis. In all cases studied, the main flux is through the PKP, while the EMP is used as a shunt. In the exponential growth phase, 70%, 73%, a ...
... both the Embden-Meyerhof pathway (EMP) and phosphoketolase pathway (PKP) when glucose or sucrose is converted into the three-carbon intermediate stage of glycolysis. In all cases studied, the main flux is through the PKP, while the EMP is used as a shunt. In the exponential growth phase, 70%, 73%, a ...
Electron Transport Chains of Lactic Acid Bacteria
... extracellular electron acceptors, via an electron transport chain (ETC) that can generate a proton motive force (PMF). This is in contrast to fermentation, defined as the oxidation of organic compounds using endogenous electron acceptors that are usually catabolic intermediates of the same organic c ...
... extracellular electron acceptors, via an electron transport chain (ETC) that can generate a proton motive force (PMF). This is in contrast to fermentation, defined as the oxidation of organic compounds using endogenous electron acceptors that are usually catabolic intermediates of the same organic c ...
... Carlsbad, CA). Plasmid pDONR221, using the BP reaction, was used to create the entry clone, designated plasmid pUD64. From this entry clone and the multicopy plasmid pAG426GPD-ccdB (Addgene, Cambridge, MA), the yeast expression plasmid pUDE43 was constructed by using the LR reaction. Transformations ...
101
... polyatomic cyanide ion, CN− . The electronegativity of nitrogen (3.04) is greater than the electronegativity of carbon (2.55). Thus, the three shared pairs of electrons are all considered to belong to the nitrogen atom. As a result, the carbon atom is considered to have two valence electrons, which ...
... polyatomic cyanide ion, CN− . The electronegativity of nitrogen (3.04) is greater than the electronegativity of carbon (2.55). Thus, the three shared pairs of electrons are all considered to belong to the nitrogen atom. As a result, the carbon atom is considered to have two valence electrons, which ...
Biological energy
... A Comparison of Chemiosmosis in Chloroplasts and Mitochondria • Chloroplasts and mitochondria – Generate ATP by the same basic mechanism: chemiosmosis – But use different sources of energy to accomplish this ...
... A Comparison of Chemiosmosis in Chloroplasts and Mitochondria • Chloroplasts and mitochondria – Generate ATP by the same basic mechanism: chemiosmosis – But use different sources of energy to accomplish this ...
Embden-Meyerhof-Parnas Pathway
... • Since NAD+ is limiting it must be regenerated (comes from niacin a vitamin). ...
... • Since NAD+ is limiting it must be regenerated (comes from niacin a vitamin). ...
Kb-Positive role of nitrite-12NoMacros
... Nitric Oxide: Biology and Chemistry. Although one of the simplest biological molecules in nature, nitric oxide has found its way into nearly every phase of biology and medicine ranging from its role as a critical endogenous regulator of blood flow and thrombosis to a principal neurotransmitter media ...
... Nitric Oxide: Biology and Chemistry. Although one of the simplest biological molecules in nature, nitric oxide has found its way into nearly every phase of biology and medicine ranging from its role as a critical endogenous regulator of blood flow and thrombosis to a principal neurotransmitter media ...
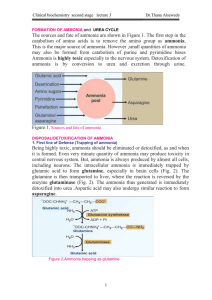
FORMATION OF AMMONIA
... results in a deficiency of one of the enzymes in the urea cycle. These enzymes are responsible for removing ammonia from the blood stream. Severe deficiency or total absence of activity of any of the first four enzymes (CPS1, OTC, ASS, ASL) in the urea cycle or the cofactor producer (NAGS) results i ...
... results in a deficiency of one of the enzymes in the urea cycle. These enzymes are responsible for removing ammonia from the blood stream. Severe deficiency or total absence of activity of any of the first four enzymes (CPS1, OTC, ASS, ASL) in the urea cycle or the cofactor producer (NAGS) results i ...
Fatty Acid Biosynthesis
... There are 4 fatty acyl desaturase enzymes in mammals designated 9 , 6, 5, and 4 fatty acyl-CoA desaturase Mammals cannot incorporate a double bond beyond 9; plants can. Mammals can synthesize long chain unsaturated fatty acids using desaturation and elongation ...
... There are 4 fatty acyl desaturase enzymes in mammals designated 9 , 6, 5, and 4 fatty acyl-CoA desaturase Mammals cannot incorporate a double bond beyond 9; plants can. Mammals can synthesize long chain unsaturated fatty acids using desaturation and elongation ...
Biochemistry - Bonham Chemistry
... • Isozymes are different enzymes that catalyze the same reaction • They typically share similar sequences • Their regulation is often different ...
... • Isozymes are different enzymes that catalyze the same reaction • They typically share similar sequences • Their regulation is often different ...
Answer Set 3
... how many protons are needed to force synthesis of one ATP: ADP + Pi → ATP + H2O? (Assume standard conditions for ATP, ADP, and Pi concentrations: ΔG’ = ΔGo’) ΔG = -2.3 RT ΔpH + z F ΔEo’ = -2.3(8.31)(298)(-1) + (n)(96,485)(0.059) = 30,500 n = 4.36 c) Alternatively, what voltage difference would be ne ...
... how many protons are needed to force synthesis of one ATP: ADP + Pi → ATP + H2O? (Assume standard conditions for ATP, ADP, and Pi concentrations: ΔG’ = ΔGo’) ΔG = -2.3 RT ΔpH + z F ΔEo’ = -2.3(8.31)(298)(-1) + (n)(96,485)(0.059) = 30,500 n = 4.36 c) Alternatively, what voltage difference would be ne ...
Pdf - Text of NPTEL IIT Video Lectures
... obviously allosteric in nature, intra cellular and it plays the different various significant roles as far as the TCA cycle is concerned. Now here, if we see this pyruvate dehydrogenase enzyme, it has got the core moieties, which is dihydrolipoyl transacetylase enzyme to which that, pyruvate dehydro ...
... obviously allosteric in nature, intra cellular and it plays the different various significant roles as far as the TCA cycle is concerned. Now here, if we see this pyruvate dehydrogenase enzyme, it has got the core moieties, which is dihydrolipoyl transacetylase enzyme to which that, pyruvate dehydro ...
Plant and Soil
... (Theodoropoulos et al., 1985), however as compared to the glucose grown cells, the glucose-dependent O2 consumption of organic acids grown cells did not exceed 25% of the maximum activity recorded (Table 3). Catabolic repression like phenomenon mediated by succinate or malate were previously observe ...
... (Theodoropoulos et al., 1985), however as compared to the glucose grown cells, the glucose-dependent O2 consumption of organic acids grown cells did not exceed 25% of the maximum activity recorded (Table 3). Catabolic repression like phenomenon mediated by succinate or malate were previously observe ...
UvA-DARE (Digital Academic Repository)
... Abstract: Modifying substrate uptake systems is a potentially powerful tool in metabolic engineering. This research investigates energetic and metabolic changes brought about by the genetic modification of the glucose uptake and phosphorylation system of Escherichia coli. The engineered strain PPA31 ...
... Abstract: Modifying substrate uptake systems is a potentially powerful tool in metabolic engineering. This research investigates energetic and metabolic changes brought about by the genetic modification of the glucose uptake and phosphorylation system of Escherichia coli. The engineered strain PPA31 ...
The urea cycle
... Since fumarate is obtained by removing NH3 from aspartate (by means of reactions 3 and 4), and PPi + H2O → 2 Pi, the equation can be simplified as follows: ...
... Since fumarate is obtained by removing NH3 from aspartate (by means of reactions 3 and 4), and PPi + H2O → 2 Pi, the equation can be simplified as follows: ...
Bacteria - McGraw Hill Higher Education
... Anaerobic metabolism by bacteria Anaerobic pathways use compounds other than O2 as terminal oxidants CH2O + NO3- CO2 + N2 or SO42-, HCO3-, Fe3+ or fumarate ...
... Anaerobic metabolism by bacteria Anaerobic pathways use compounds other than O2 as terminal oxidants CH2O + NO3- CO2 + N2 or SO42-, HCO3-, Fe3+ or fumarate ...
Chapter 23
... double bond, and reduction of the alcoholic carbon to a ketone by an NAD +-dependent enzyme. In -oxidation (Figure 23.10), a similar series operates but with an acyl-CoA as substrate and thiolysis of the product to release acetyl-CoA and a fatty acyl-CoA, two carbons shorter. The FAD-dependent enzy ...
... double bond, and reduction of the alcoholic carbon to a ketone by an NAD +-dependent enzyme. In -oxidation (Figure 23.10), a similar series operates but with an acyl-CoA as substrate and thiolysis of the product to release acetyl-CoA and a fatty acyl-CoA, two carbons shorter. The FAD-dependent enzy ...
Microbial Production of Organic Acids
... Pasteur in the latter part of the nineteenth century. **The breaking down of complex organic substances into simpler ones. Fermentation is a metabolic process that converts sugar to acids, gases and/or alcohol. It occurs in yeast and bacteria, but also in oxygen-starved muscle cells, as in the case ...
... Pasteur in the latter part of the nineteenth century. **The breaking down of complex organic substances into simpler ones. Fermentation is a metabolic process that converts sugar to acids, gases and/or alcohol. It occurs in yeast and bacteria, but also in oxygen-starved muscle cells, as in the case ...
Pyruvate dehydrogenase
... pathway occurring in plants and several bacteria, but not animals. . The glyoxylate cycle allows these organisms to use fats for the synthesis of carbohydrates, a task which vertebrates, including humans, cannot perform. Isocitrate --> succinate + glyoxylate (O=CH-COO-)+acetyl-CoA--> malate-->> gluc ...
... pathway occurring in plants and several bacteria, but not animals. . The glyoxylate cycle allows these organisms to use fats for the synthesis of carbohydrates, a task which vertebrates, including humans, cannot perform. Isocitrate --> succinate + glyoxylate (O=CH-COO-)+acetyl-CoA--> malate-->> gluc ...
Topic 9 Oxidation and Reduction Answers - slider-dpchemistry-11
... Rule/s: Three rules are used here. Firstly, hydrogen always has an oxidation of +1 (except in combination with reactive metals such as Na when it is -1). Secondly, oxygen always has an oxidation state of –2 (except in H2O2 where it is -1). These known values are used first. Finally, as all these mol ...
... Rule/s: Three rules are used here. Firstly, hydrogen always has an oxidation of +1 (except in combination with reactive metals such as Na when it is -1). Secondly, oxygen always has an oxidation state of –2 (except in H2O2 where it is -1). These known values are used first. Finally, as all these mol ...
Scheme of Metabolism
... ~ ~ + Reducing+ Yields power ATP substrate-level phosphorylation Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. ...
... ~ ~ + Reducing+ Yields power ATP substrate-level phosphorylation Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. ...
Microbial metabolism
Microbial metabolism is the means by which a microbe obtains the energy and nutrients (e.g. carbon) it needs to live and reproduce. Microbes use many different types of metabolic strategies and species can often be differentiated from each other based on metabolic characteristics. The specific metabolic properties of a microbe are the major factors in determining that microbe’s ecological niche, and often allow for that microbe to be useful in industrial processes or responsible for biogeochemical cycles.== Types of microbial metabolism ==All microbial metabolisms can be arranged according to three principles:1. How the organism obtains carbon for synthesising cell mass: autotrophic – carbon is obtained from carbon dioxide (CO2) heterotrophic – carbon is obtained from organic compounds mixotrophic – carbon is obtained from both organic compounds and by fixing carbon dioxide2. How the organism obtains reducing equivalents used either in energy conservation or in biosynthetic reactions: lithotrophic – reducing equivalents are obtained from inorganic compounds organotrophic – reducing equivalents are obtained from organic compounds3. How the organism obtains energy for living and growing: chemotrophic – energy is obtained from external chemical compounds phototrophic – energy is obtained from lightIn practice, these terms are almost freely combined. Typical examples are as follows: chemolithoautotrophs obtain energy from the oxidation of inorganic compounds and carbon from the fixation of carbon dioxide. Examples: Nitrifying bacteria, Sulfur-oxidizing bacteria, Iron-oxidizing bacteria, Knallgas-bacteria photolithoautotrophs obtain energy from light and carbon from the fixation of carbon dioxide, using reducing equivalents from inorganic compounds. Examples: Cyanobacteria (water (H2O) as reducing equivalent donor), Chlorobiaceae, Chromatiaceae (hydrogen sulfide (H2S) as reducing equivalent donor), Chloroflexus (hydrogen (H2) as reducing equivalent donor) chemolithoheterotrophs obtain energy from the oxidation of inorganic compounds, but cannot fix carbon dioxide (CO2). Examples: some Thiobacilus, some Beggiatoa, some Nitrobacter spp., Wolinella (with H2 as reducing equivalent donor), some Knallgas-bacteria, some sulfate-reducing bacteria chemoorganoheterotrophs obtain energy, carbon, and reducing equivalents for biosynthetic reactions from organic compounds. Examples: most bacteria, e. g. Escherichia coli, Bacillus spp., Actinobacteria photoorganoheterotrophs obtain energy from light, carbon and reducing equivalents for biosynthetic reactions from organic compounds. Some species are strictly heterotrophic, many others can also fix carbon dioxide and are mixotrophic. Examples: Rhodobacter, Rhodopseudomonas, Rhodospirillum, Rhodomicrobium, Rhodocyclus, Heliobacterium, Chloroflexus (alternatively to photolithoautotrophy with hydrogen)