* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Microbial Production of Organic Acids
Survey
Document related concepts
Microbial metabolism wikipedia , lookup
Peptide synthesis wikipedia , lookup
Metalloprotein wikipedia , lookup
Genetic code wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Biosynthesis wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
15-Hydroxyeicosatetraenoic acid wikipedia , lookup
Biochemistry wikipedia , lookup
Specialized pro-resolving mediators wikipedia , lookup
Citric acid cycle wikipedia , lookup
Transcript
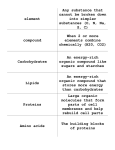
Microbial Production of Organic Acids Zulal ÖZDEMIR Institute of Chemical Technology, Prague Faculty of Food and Biochemical Technology, Department of Biochemistry and Microbiology 166 28, Praha 6 CZECH REPUBLIC May, 2014 Microbial products often classified as primary and secondary metabolites. Primary metabolites are considered essential to microorganisms for proper growth. They include amino acids, nucleotides, and fermentation end products such as ethanol and organic acids. Secondary metabolites do not play a role in growth, development, and reproduction, and are formed during the end or near the stationary phase of growth. Most antibiotics and the mycotoxins fall into this category. What are the organic acids? Organic acids are organic compounds with acidic properties. The most common organic acids are the carboxylic acids whose acidity is associated with their carboxyl group -COOH. Organic acids have been used as Food additives Preservatives for preventing food deterioration and extending the shelf life of perishable food ingredient. The organic acids produced by various microbes via fermentation Figure 1. Diagram of the Carboxylic acid group What is fermentation? Fermentation has always been an important part of our lives: Foods can be spoiled by microbial fermentations, Foods can be made by microbial fermentations, (alcoholic beverages or acidic dairy products.) Muscle cells use fermentation to provide us with quick responses.(way of getting energy without using oxygen) But how fermentation actually works was not understood until the work of Louis Pasteur in the latter part of the nineteenth century. **The breaking down of complex organic substances into simpler ones. Fermentation is a metabolic process that converts sugar to acids, gases and/or alcohol. It occurs in yeast and bacteria, but also in oxygen-starved muscle cells, as in the case of lactic acid fermentation. What are advantages of fermentation over chemical sythesis for production of organic acids? The chemical synthesis of organic acids : Requires very harsh conditions Involves many steps which make their large scale production impractical. The microbial fermentation is: A very simple method to synthesize these organic acids in very pure form. Require less energy input and are cost effective due to simple media formulations in many cases. All acids of the tricarboxylic acid (TCA) cycle can be produced microbially in high yields(citric acid), other acids can be derived indirectly from the Krebs cycle such as itaconic acid, or can be derived directly from glucose (gluconic acid). Some acids are formed as the end products from pyruvate or ethanol (lactic and acetic acid). Large-scale commercial production of a number of organic acids are citricgluconic- and itaconic acid. Other organic acids produced in lower scale are lactic acid, malic acid, gibberellic acid, and kojic acid. Citric acid is by far the major organic acid in worldwide production. Table 1. Worldwide organic acid production Citric Acid Citric acid (2-hydroxypropane-1,2,3-tricarboxylic acid) is one of the world’s major fermentation products with the annual production of over 550,000 tonnes, and its demand is increasing at the rate of 2-3% every year. It was first isolated in 1784 from lemon juice and crystallized by Scheele. Until the 1920s, citric acid was extracted from the lemon juice and referred as ‘natural citric acid’. Whehmer first time in 1923 described that citric acid is a metabolic product of Penicillium and Mucor. In 1923, Pfizer became the first industry to produce citric acid through fermentation based process in USA, by culturing Aspergillus niger in surface culture in a medium containing sucrose and mineral salts. As on today most of the citric acid is produced by fermentation, and the major producers are located in Western Europe, USA and China. Figure 2. Structure of Citric acid Produced by many microorganisms including filamentus fungi, yeasts and bacteria. All of them could be used to produce citric acid, however, the mutants of A. niger are generally used for commercial use.. Carbohydrate sources such as beet molasses, sucrose, commercial glucose, starch hydrolysis etc. The raw material is diluted to 20-25 per cent sugar concentration and mixed with nitrogen source and other salts. The pH of the medium is maintained around 5.0, when molasses are used and pH is adjusted at 3.0 when sucrose is used. The fermentation is carried out either under the surface, submerged or under solid state conditions. Citric acid fermentation by Aspergillus niger is greatly enhanced in the presence of trace metals such as iron, zinc, copper, manganese etc. in the medium.. Table 2. Microorganisms which produce citric acid The first stages of citric acid formation involve the breakdown of hexoses to pyruvate in glycolysis, followed by its decarboxylation to produce acetyl CoA CO2 released during this reaction is not lost, but is recycled by pyruvate carboxylase which is produced constitutively in Aspergillus. Normally, oxaloacetate would largely be supplied through the completion of the TCA cycle, allowing recommencement of the cycle by condensing with acetyl CoA to form citrate, catalysed by citrate synthase. However, in order to accumulate citrate, continuation of the cycle must be blocked. This is achieved by inhibiting aconitase, the enzyme catalysing the next step in the TCA cycle. Inhibition is accomplished by removal of iron, an activator of aconitase. Consequently, during citrate accumulation, the TCA cycle is largely inoperative beyond citrate formation. Colonies of Aspergillus niger The black dots covering the colonies are Aspergillus spores. The growth medium is Saboraud's Dextrose Agar (SDA) Citric acid has GRAS (generally regarded as safe) status and its major applications are summarized below Lactic Acid Lactic acid (2-hydroxypropanoic acid) was discovered and isolated in 1780 by the Swedish chemist Scheele from sour milk and the first organic acid produced microbiologically in 1881 by Charles E. Avery at Littleton, Massachusetts, USA. It is classified as GRAS (generally regarded as safe) by Food and Drug Authority (FDA) in the USA and its annual consumption is estimated to be 30 000 tonnes. Lactic acid is used in various industries for different applications Figure 3. Structure of lactic acid Major applications of lactic acid are summarized below Lactic acid bacterias There are two groups of lactic acid bacteria, one is heterofermentative and other is homofermentative. The heterofermentative (e.g. Luconostoc mesenteroides) lactic acid bacteria produce many byproducts other than lactic acid and are not suitable for commercial processes. The homofermentative bacteria (Lactobacillus sp.), very little substrate is used for producing cell mass and other metabolites and majority of the carbon source is converted into lactic acid. Some Lactobacillus species and their preferred carbon sources Lactic acid fermentation Used carbohydrates: corn starch, potato starch, molasses and whey. When starchy materials are used, they are initially hydrolysed to simple sugars. The medium is supplemented with a nitrogen source and calcium carbonate and the fermentation is carried out by inoculation with homofermentative Lactobacilli. During the fermentation the temperature is controlled at 40-50° C depending on the organism. The medium is kept in constant agitation. After completion of the fermentation for 4-6 days, the fermented liquor is heated to 82°C and then, filtration is done. Itaconic Acid In 1931, itaconic acid was first shown to be a metabolic product of Aspergillus itaconicus and soon after, it was discovered that some strains of A. terreus also excrete this type of organic acid. In today’s commercial production processes, mutants of both strains are used. Formed in a branch of the TCA cycle via decarboxylation of cis-aconitate which is normally followed by its oxidation to itatartaric acid. Onward metabolism of itaconic acid must be prevented in commercial fermentations, otherwise yield is reduced. This is achieved by formulating the medium with high levels of calcium ions, thereby inhibiting itaconic acid oxidase, which catalyses the oxidation of itaconic acid to itatartaric acid. Figure 4. Structure of lactic acid Acetic Acid Acetic acid production from alcoholic liquids has been carried out for nearly 10,000 years. The Romans and the Greeks used diluted vinegar as a refreshing drink, produced the vinegar by leaving wine open to the air. The first industrially manufactured vinegars were produced in flat open vats. This process involved a film of bacteria forming over the surface of the wine. This was a very slow process and it wasn’t until the nineteenth century that surface fermentations were developed into more rapid procedures. Many fermentative bacteria produce acetic acid, but only members of the acetic acid bacteria group are used in commercial production. This group can be divided into two genera, Gluconobacter and Acetobacter. Figure 5. Structrure of acetic acid Acetic Acid Fermentation Gluconobacter: Oxidizes ethanol solely to acetic acid. Members of the genus Gluconobacter are not over oxidizers. Mixed cultures appear during production, even when the inoculum is assumed pure. Acetobacter: Oxidize ethanol first to acetic acid and then further to CO2 and H2O. Members of the genus Acetobacter are gram negative and acid tolerant. Gluconic Acid Gluconic acid (2,3,4,5,6-Pentahydroxycaproic acid) is used in the food and beverage, pharmaceutical, detergent and construction industries. It is produced using the organism Aspergillus niger. The growth medium in this fermentation is based on glucose and corn steep liquor with other nutritional requirements including ammonium salts and urea. Too much nitrogen leads to excessive growth of organism therefore producing lower acid yields. The production pH is maintained at 6.0-7.0 until optimum growth and a set glucose oxidase level has been achieved. After this point, the pH may be allowed to drop to 3.5. The temperature is maintained throughout the process at 33°C. This fermentation requires a vast amount of aeration, as the process is highly aerobic. Figure 6. Structrure of gluconic acid Gluconic Acid Fermentation Gluconic acid is produced extracellularly! The spontaneous conversion of β-D-Glucose from α-D-Glucose is accelerated by the enzyme mutarose in A. niger, β-D-Glucose is converted to D-glucono-δ-lactone by glucose oxidase. This enzyme is induced by glucose presence at pH values >4 and is denatured below pH 2. The conversion of D-glucono-δ-lactone to D-Gluconic acid is spontaneous at neutral pH. At lower pH, this conversion is less effective and facilitated by D-glucono-δ-lactonase by A. niger. This enzymatic process has the added advantage that no product purification steps are required after the fermentation. Thank you for your attention!


































![fermentation[1].](http://s1.studyres.com/store/data/008290469_1-3a25eae6a4ca657233c4e21cf2e1a1bb-150x150.png)