CHEMISTRY OF FOOD FERMENTATION
... a rare medical condition where the stomach produce brewer’s yeast that break down starches into ethanol; which enters the blood stream. Fermentation is a form of anaerobic digestion that generates adenosine triphosphate (ATP) by the process of substrate-level phosphorylation. The energy for generati ...
... a rare medical condition where the stomach produce brewer’s yeast that break down starches into ethanol; which enters the blood stream. Fermentation is a form of anaerobic digestion that generates adenosine triphosphate (ATP) by the process of substrate-level phosphorylation. The energy for generati ...
Guided Reading Unit 3
... What kind of fermentation do humans do? During lactic acid fermentation, pyruvate is reduced to: How is this fermentation used by industry? ...
... What kind of fermentation do humans do? During lactic acid fermentation, pyruvate is reduced to: How is this fermentation used by industry? ...
Macromolecule Packet
... 23. Amino acids are linked together to make proteins by removing a molecule of ________ in a process called ____________. 24. Chains of amino acids make _______________ which can join together to make a __________. 25. __________ bonds form when water is removed to hold _________ acids together. Lip ...
... 23. Amino acids are linked together to make proteins by removing a molecule of ________ in a process called ____________. 24. Chains of amino acids make _______________ which can join together to make a __________. 25. __________ bonds form when water is removed to hold _________ acids together. Lip ...
SOIL BIOLOGY AND ECOLOGY
... They are like bacteria in that they are single celled and about same size, and have no nuclear membrane. They are heterotrophs that live mostly on decaying organic matter. They develop best in moist, well aerated soil (do poorly below pH 6). -biomass ~ 5000 kg/ha Actinomycetes produce antibiotic ...
... They are like bacteria in that they are single celled and about same size, and have no nuclear membrane. They are heterotrophs that live mostly on decaying organic matter. They develop best in moist, well aerated soil (do poorly below pH 6). -biomass ~ 5000 kg/ha Actinomycetes produce antibiotic ...
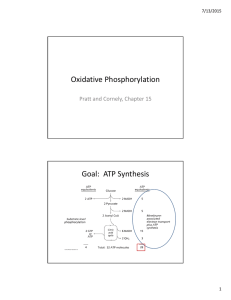
Oxidative Phosphorylation Goal: ATP Synthesis
... – The pH of the intermembrane space is lower than the pH of the mitochondrial matrix. – Oxidative phosphorylation does not occur in mitochondrial preparations to which detergents have been added. – Lipid‐soluble compounds inhibit oxidative phosphorylation while allowing electron transport to co ...
... – The pH of the intermembrane space is lower than the pH of the mitochondrial matrix. – Oxidative phosphorylation does not occur in mitochondrial preparations to which detergents have been added. – Lipid‐soluble compounds inhibit oxidative phosphorylation while allowing electron transport to co ...
Cell Respiration - Biology Junction
... 3) turns twice because two acetyl-CoA molecules enter the cycle per glucose molecule; 4) produces two immediate ATP molecules per glucose molecule. d. The electron transport chain: 1) is a series of carriers in the inner mitochondrial membrane that accept electrons from glucose--electrons are passed ...
... 3) turns twice because two acetyl-CoA molecules enter the cycle per glucose molecule; 4) produces two immediate ATP molecules per glucose molecule. d. The electron transport chain: 1) is a series of carriers in the inner mitochondrial membrane that accept electrons from glucose--electrons are passed ...
Energy for Muscle Contractions
... Creatine Phosphate This is a high energy compound used to produce ATP – Provides for about 15 seconds of maximal contraction Chemical equation = ...
... Creatine Phosphate This is a high energy compound used to produce ATP – Provides for about 15 seconds of maximal contraction Chemical equation = ...
Glycolysis and Anaerobic Respiration Lecture Notes
... • The products of glycolysis can be further broken down without the use of oxygen through additional biochemical pathways that occur in the cytosol. • The combination of glycolysis pulse these additional pathways is called fermentation. • Fermentation does not produce any additional ATP but it does ...
... • The products of glycolysis can be further broken down without the use of oxygen through additional biochemical pathways that occur in the cytosol. • The combination of glycolysis pulse these additional pathways is called fermentation. • Fermentation does not produce any additional ATP but it does ...
organism
... 3. Name an abiotic factor. Explain how a change in an abiotic factor would affect biodiversity. ...
... 3. Name an abiotic factor. Explain how a change in an abiotic factor would affect biodiversity. ...
UNIT 2 Ecology - Winston Knoll Collegiate
... o Evaporation changes surface water (lakes, rivers, oceans) to water vapor • Water vapor (gaseous state) returns to the atmosphere o Transpiration is the loss of water vapor from the leaves of plants • Stomata are openings in leaves which allow the water vapor out of the plant ...
... o Evaporation changes surface water (lakes, rivers, oceans) to water vapor • Water vapor (gaseous state) returns to the atmosphere o Transpiration is the loss of water vapor from the leaves of plants • Stomata are openings in leaves which allow the water vapor out of the plant ...
Abstract_Metabolomic_RFMF
... By definition, metabolites are intermediates and products from different metabolisms. More commonly, they are small compounds (between 100 and 1000 Da) found in organisms that have an important part in cells live and survival. They can be identified with different technics such as Nuclear Magnetic R ...
... By definition, metabolites are intermediates and products from different metabolisms. More commonly, they are small compounds (between 100 and 1000 Da) found in organisms that have an important part in cells live and survival. They can be identified with different technics such as Nuclear Magnetic R ...
Stryer An overview of the citric acid cycle
... 2) the respiratory chain as an energy source 3) oxidative phosphorylation and uncouplers 4) membrane transporters and shuttles a) cytosolic NADH oxidation ...
... 2) the respiratory chain as an energy source 3) oxidative phosphorylation and uncouplers 4) membrane transporters and shuttles a) cytosolic NADH oxidation ...
NAME: : :______ Honors Biology Reading Guide – Chapter 6
... 69. One mole of glucose stores _____kcal of energy which is then utilized by living things when carbohydrates are oxidized in ______________________________________________. Mitochondria and Cellular Respiration 70. Write the overall equation for cellular respiration. ...
... 69. One mole of glucose stores _____kcal of energy which is then utilized by living things when carbohydrates are oxidized in ______________________________________________. Mitochondria and Cellular Respiration 70. Write the overall equation for cellular respiration. ...
Cellular Respiration
... • Up to 36 ATP molecules can be produced from one glucose molecule in aerobic respiration. ...
... • Up to 36 ATP molecules can be produced from one glucose molecule in aerobic respiration. ...
Energy Flow - SchoolRack
... – a. Demonstrate in a food web that matter is transferred from one organism to another and can recycle between organisms and their environments. – b. Explain in a food web that sunlight is the source of energy and that this energy moves from organism to organism. – c. Recognize that changes in envir ...
... – a. Demonstrate in a food web that matter is transferred from one organism to another and can recycle between organisms and their environments. – b. Explain in a food web that sunlight is the source of energy and that this energy moves from organism to organism. – c. Recognize that changes in envir ...
Biomolecules review with answers
... molecules used for energy during respiration. 28. How are starch and glycogen the same? How are they different? Both are polymers of sugars. Starch is a long and sometimes slight branched chain of sugars. Glycogen is high branched. 29. How are starch and cellulose the same? How are they different? B ...
... molecules used for energy during respiration. 28. How are starch and glycogen the same? How are they different? Both are polymers of sugars. Starch is a long and sometimes slight branched chain of sugars. Glycogen is high branched. 29. How are starch and cellulose the same? How are they different? B ...
UNIT 10 TEXT WS: “Organic Chemistry”
... What types of bonds are present in each of these classes of organic compounds? What ratio of bonded carbon and hydrogen atoms do simple examples of each of these follow? Types of Bonds ...
... What types of bonds are present in each of these classes of organic compounds? What ratio of bonded carbon and hydrogen atoms do simple examples of each of these follow? Types of Bonds ...
1.Oxidative phosphorylation
... tissue is abundant in the newborn and in some adult mammals, and it is brown because of its high content of mitochondria. In humans, brown adipose tissue is abundant in infants, but it gradually diminishes and is barely detectable in adults. • UCP1 provides body heat during cold stress in the young ...
... tissue is abundant in the newborn and in some adult mammals, and it is brown because of its high content of mitochondria. In humans, brown adipose tissue is abundant in infants, but it gradually diminishes and is barely detectable in adults. • UCP1 provides body heat during cold stress in the young ...
Chapter 8 THE ENERGY CONSUMING PROCESS OF RESPIRATION
... The direction H+ ion diffusion is indicated by the location of ATP synthase proteins (lollipops). Mictochondria and some bacteria use chemiosmosis to make ATP during cell respiration. Chloroplasts use chemiosmosis to make ATP as part of photosynthsis. ...
... The direction H+ ion diffusion is indicated by the location of ATP synthase proteins (lollipops). Mictochondria and some bacteria use chemiosmosis to make ATP during cell respiration. Chloroplasts use chemiosmosis to make ATP as part of photosynthsis. ...
Slides - gserianne.com
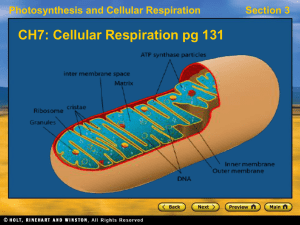
... Occurs in three major of reaction series… 1. Glycolysis (glucose to pyruvate; in cytoplasm) 2. Citric acid cycle (finishes oxidation begun in glycolysis; in the matrix of mitochondria) 3. Electron transport chain (uses e- transfer to make ATP; on inner membranes of mitochondria) Produces • carbon di ...
... Occurs in three major of reaction series… 1. Glycolysis (glucose to pyruvate; in cytoplasm) 2. Citric acid cycle (finishes oxidation begun in glycolysis; in the matrix of mitochondria) 3. Electron transport chain (uses e- transfer to make ATP; on inner membranes of mitochondria) Produces • carbon di ...
Microbial metabolism
Microbial metabolism is the means by which a microbe obtains the energy and nutrients (e.g. carbon) it needs to live and reproduce. Microbes use many different types of metabolic strategies and species can often be differentiated from each other based on metabolic characteristics. The specific metabolic properties of a microbe are the major factors in determining that microbe’s ecological niche, and often allow for that microbe to be useful in industrial processes or responsible for biogeochemical cycles.== Types of microbial metabolism ==All microbial metabolisms can be arranged according to three principles:1. How the organism obtains carbon for synthesising cell mass: autotrophic – carbon is obtained from carbon dioxide (CO2) heterotrophic – carbon is obtained from organic compounds mixotrophic – carbon is obtained from both organic compounds and by fixing carbon dioxide2. How the organism obtains reducing equivalents used either in energy conservation or in biosynthetic reactions: lithotrophic – reducing equivalents are obtained from inorganic compounds organotrophic – reducing equivalents are obtained from organic compounds3. How the organism obtains energy for living and growing: chemotrophic – energy is obtained from external chemical compounds phototrophic – energy is obtained from lightIn practice, these terms are almost freely combined. Typical examples are as follows: chemolithoautotrophs obtain energy from the oxidation of inorganic compounds and carbon from the fixation of carbon dioxide. Examples: Nitrifying bacteria, Sulfur-oxidizing bacteria, Iron-oxidizing bacteria, Knallgas-bacteria photolithoautotrophs obtain energy from light and carbon from the fixation of carbon dioxide, using reducing equivalents from inorganic compounds. Examples: Cyanobacteria (water (H2O) as reducing equivalent donor), Chlorobiaceae, Chromatiaceae (hydrogen sulfide (H2S) as reducing equivalent donor), Chloroflexus (hydrogen (H2) as reducing equivalent donor) chemolithoheterotrophs obtain energy from the oxidation of inorganic compounds, but cannot fix carbon dioxide (CO2). Examples: some Thiobacilus, some Beggiatoa, some Nitrobacter spp., Wolinella (with H2 as reducing equivalent donor), some Knallgas-bacteria, some sulfate-reducing bacteria chemoorganoheterotrophs obtain energy, carbon, and reducing equivalents for biosynthetic reactions from organic compounds. Examples: most bacteria, e. g. Escherichia coli, Bacillus spp., Actinobacteria photoorganoheterotrophs obtain energy from light, carbon and reducing equivalents for biosynthetic reactions from organic compounds. Some species are strictly heterotrophic, many others can also fix carbon dioxide and are mixotrophic. Examples: Rhodobacter, Rhodopseudomonas, Rhodospirillum, Rhodomicrobium, Rhodocyclus, Heliobacterium, Chloroflexus (alternatively to photolithoautotrophy with hydrogen)