Inorganic vs. Organic Compounds Carbon Compounds Polymerize
... – Common examples: fats, oils, and waxes. – Lipids are made of C, H, and O (no ratio H to O). – Lipids function in energy storage, form biological membranes, and act as chemical messengers. Lipids have more energy than carbohydrates because lipids have more hydrogens bonded to the carbon chain. ...
... – Common examples: fats, oils, and waxes. – Lipids are made of C, H, and O (no ratio H to O). – Lipids function in energy storage, form biological membranes, and act as chemical messengers. Lipids have more energy than carbohydrates because lipids have more hydrogens bonded to the carbon chain. ...
Lesson element
... This is equal to the amount of carbon dioxide produced divided by the amount of oxygen produced. For a carbohydrate RQ is 1 during aerobic respiration. In anaerobic respiration, only carbon dioxide is produced and no oxygen used and so, if the two are occurring together, then the RQ may rise above 1 ...
... This is equal to the amount of carbon dioxide produced divided by the amount of oxygen produced. For a carbohydrate RQ is 1 during aerobic respiration. In anaerobic respiration, only carbon dioxide is produced and no oxygen used and so, if the two are occurring together, then the RQ may rise above 1 ...
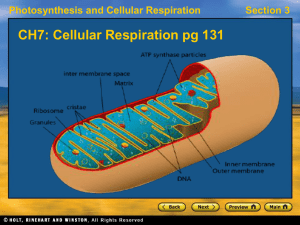
CH7Cellular-Respiration
... Efficiency of Cellular Respiration • In the first stage of cellular respiration, glucose is broken down to pyruvate during glycolysis, an anaerobic process. • Glycolysis results in 2 ATP molecules for each glucose molecule that is broken down. • In the 2nd stage, pyruvate EITHER passes through the ...
... Efficiency of Cellular Respiration • In the first stage of cellular respiration, glucose is broken down to pyruvate during glycolysis, an anaerobic process. • Glycolysis results in 2 ATP molecules for each glucose molecule that is broken down. • In the 2nd stage, pyruvate EITHER passes through the ...
gil, virginia
... and form ionic bonds; it would have to donate or accept for electrons to do so. Instead, a carbon atom completes its valence shell by sharing electrons with other atoms in four covalent bonds. Each carbon thus acts as an intersection point from which a molecule can branch off in up to four direction ...
... and form ionic bonds; it would have to donate or accept for electrons to do so. Instead, a carbon atom completes its valence shell by sharing electrons with other atoms in four covalent bonds. Each carbon thus acts as an intersection point from which a molecule can branch off in up to four direction ...
Document
... • Recent finding suggest that the control of most pathways is shared by multiple pathway enzymes ...
... • Recent finding suggest that the control of most pathways is shared by multiple pathway enzymes ...
Limits of Human Performance
... – Sum total of all chemical processes within an organism; produces heat. Why? – Metabolic rate: can be measured as heat production – O2 consumption provides for almost all of our metabolic needs, so Vo2 provides a very good index of metabolic rate – High Vo2 means high metabolic capacity ...
... – Sum total of all chemical processes within an organism; produces heat. Why? – Metabolic rate: can be measured as heat production – O2 consumption provides for almost all of our metabolic needs, so Vo2 provides a very good index of metabolic rate – High Vo2 means high metabolic capacity ...
Cellular Respiration
... Why can cells obtain energy from oxidising molecules such as glucose? The carbon and hydrogen atoms in cells, for example in glucose molecules, are not in their most stable form The most energetically stable form of carbon is carbon dioxide and the most energetically stable form of hydrogen is ...
... Why can cells obtain energy from oxidising molecules such as glucose? The carbon and hydrogen atoms in cells, for example in glucose molecules, are not in their most stable form The most energetically stable form of carbon is carbon dioxide and the most energetically stable form of hydrogen is ...
Photosynthesis
... the chloroplast) from the surrounding cytosol of the cell The carbon dioxide then goes through a series of “cycles” to create organic compounds ...
... the chloroplast) from the surrounding cytosol of the cell The carbon dioxide then goes through a series of “cycles” to create organic compounds ...
Chapter 5:Bioenergetics and oxidative phosphorylation Q1: why is
... Q3: what are the biochemical uses of NADPH? Q4: List down the antioxidant enzyme? What is the specific reaction catalyzed by each enzyme? Q5: what is the structure of glutathione? Q6: How is reduced glutathione (0-OH) regenerated? Q7: what is the substrate of glutathione? Q8: why are RBCs more susce ...
... Q3: what are the biochemical uses of NADPH? Q4: List down the antioxidant enzyme? What is the specific reaction catalyzed by each enzyme? Q5: what is the structure of glutathione? Q6: How is reduced glutathione (0-OH) regenerated? Q7: what is the substrate of glutathione? Q8: why are RBCs more susce ...
Carbon transfer from dissolved organic carbon to the cladoceran
... (Rösel et al., 2012). Second, the presence of mixotrophic and heterotrophic protists by grazing on bacteria or osmosis as well as osmotrophic algae, which are able to assimilate DOC and synthesize HUFA (Jones, 2000; Tittel et al., 2009). A third possibility is that dissolved inorganic carbon (DIC) c ...
... (Rösel et al., 2012). Second, the presence of mixotrophic and heterotrophic protists by grazing on bacteria or osmosis as well as osmotrophic algae, which are able to assimilate DOC and synthesize HUFA (Jones, 2000; Tittel et al., 2009). A third possibility is that dissolved inorganic carbon (DIC) c ...
6 Aerobic Degradation by Microorganisms
... (3) Peripheral degradation pathways convert organic pollutants step by step into intermediates of the central intermediary metabolism, e.g., the tricarboxylic acid cycle. (4) Biosynthesis of cell biomass from the central precursor metabolites, e.g., acetyl-CoA, succinate, pyruvate. Sugars required f ...
... (3) Peripheral degradation pathways convert organic pollutants step by step into intermediates of the central intermediary metabolism, e.g., the tricarboxylic acid cycle. (4) Biosynthesis of cell biomass from the central precursor metabolites, e.g., acetyl-CoA, succinate, pyruvate. Sugars required f ...
1 Everything Is Connected
... how different organisms interact with one another and their environment is ecology. An alligator may hunt along the edge of a river. It may catch a fish, such as a gar, that swims by too closely. As it hunts, the alligator is interacting with its environment. Its environment includes other organisms ...
... how different organisms interact with one another and their environment is ecology. An alligator may hunt along the edge of a river. It may catch a fish, such as a gar, that swims by too closely. As it hunts, the alligator is interacting with its environment. Its environment includes other organisms ...
Energy Metabolism - Georgia Institute of Technology
... – H+ actively transported out of matrix – H+ leak back as H+PO4 2- ...
... – H+ actively transported out of matrix – H+ leak back as H+PO4 2- ...
Fermentation - cloudfront.net
... Name the two types of fermentation. How much ATP does glycolysis create? How much ATP does fermentation create? Which molecule is broken down during glycolysis? A buildup of which molecule causes sore muscles? Which waste molecules are created by alcoholic fermentation? 7) Is fermentation aerobic or ...
... Name the two types of fermentation. How much ATP does glycolysis create? How much ATP does fermentation create? Which molecule is broken down during glycolysis? A buildup of which molecule causes sore muscles? Which waste molecules are created by alcoholic fermentation? 7) Is fermentation aerobic or ...
doc: Oxidation Numbers
... Oxidation Numbers It is often useful to follow chemical reactions by looking at changes in the oxidation numbers of the atoms in each compound during the reaction. Oxidation numbers also play an important role in the naming of chemical compounds. By definition, the oxidation number of an atom is the ...
... Oxidation Numbers It is often useful to follow chemical reactions by looking at changes in the oxidation numbers of the atoms in each compound during the reaction. Oxidation numbers also play an important role in the naming of chemical compounds. By definition, the oxidation number of an atom is the ...
Glucose or Ethanol
... Alcoholic fermentations, Example: wine or beer fermentations AEROBIC (In the presence of Oxygen) Yeast propagation ...
... Alcoholic fermentations, Example: wine or beer fermentations AEROBIC (In the presence of Oxygen) Yeast propagation ...
BSU Reading Guide Chapter 7 Respiration
... Ethanol Fermentation Eukaryotic cells are capable of only a few types of fermentation. In one type, which occurs in single-celled fungi called yeast, the molecule that accepts hydrogen from NADH is pyruvate, the end product of glycolysis itself. Yeast enzymes remove a CO2 group from pyruvate through ...
... Ethanol Fermentation Eukaryotic cells are capable of only a few types of fermentation. In one type, which occurs in single-celled fungi called yeast, the molecule that accepts hydrogen from NADH is pyruvate, the end product of glycolysis itself. Yeast enzymes remove a CO2 group from pyruvate through ...
First Homework Assignment
... galactose. There are four possible connection spots to glucose (2, 3, 4, or 6-1 will be discussed later) and each connecting galactose can be in either the or type of glycosidic linkage. Ignoring the free reducing end of glucose (which will be in equilibrium between the two anomers) this gives 8 ...
... galactose. There are four possible connection spots to glucose (2, 3, 4, or 6-1 will be discussed later) and each connecting galactose can be in either the or type of glycosidic linkage. Ignoring the free reducing end of glucose (which will be in equilibrium between the two anomers) this gives 8 ...
3 H 2 - KMIT
... threads. Signals stimulate cell multiplication of both the plant's cells and the bacteria and this repeated division results in a mass of root cells containing many bacterial cells. Some of these bacteria then change into a form that is able to convert gaseous nitrogen into ammonium nitrogen (that i ...
... threads. Signals stimulate cell multiplication of both the plant's cells and the bacteria and this repeated division results in a mass of root cells containing many bacterial cells. Some of these bacteria then change into a form that is able to convert gaseous nitrogen into ammonium nitrogen (that i ...
Biology CP
... Identify primary, secondary and tertiary consumers and producers Explain how energy is passed from one trophic level to the next Calculate how much energy is passed from one trophic level to the next Predict how an ecosystem will be affected by certain organisms being Killed off Be able to interpret ...
... Identify primary, secondary and tertiary consumers and producers Explain how energy is passed from one trophic level to the next Calculate how much energy is passed from one trophic level to the next Predict how an ecosystem will be affected by certain organisms being Killed off Be able to interpret ...
BI0 120 cell and tissues
... 28. The activation phase of the glycolysis consist of A. adding phosphates, modifying sugars and forming glyceraldehyde-3-phosphate. B. oxidative steps, proton pumping, and reaction with oxygen. C. oxidation of glyceraldehyde-3-phosphate, and storage of energy. D. ATP synthesis by substrate-level ph ...
... 28. The activation phase of the glycolysis consist of A. adding phosphates, modifying sugars and forming glyceraldehyde-3-phosphate. B. oxidative steps, proton pumping, and reaction with oxygen. C. oxidation of glyceraldehyde-3-phosphate, and storage of energy. D. ATP synthesis by substrate-level ph ...
Basic Concepts of Cellular Metabolism and Bioenergetics
... fatty acids and glycerol. Moves materials into blood for transport to cells. ...
... fatty acids and glycerol. Moves materials into blood for transport to cells. ...
LAB 6 – Fermentation & Cellular Respiration INTRODUCTION
... (the electrons being “carried” are associated with the hydrogen atom) during glycolysis. Fermentation is simply one or more biochemical steps that transfer the H in NADH and an extra electron to a molecule of pyruvate. As a result, NADH is restored to NAD+, which is needed for glycolysis, and pyruva ...
... (the electrons being “carried” are associated with the hydrogen atom) during glycolysis. Fermentation is simply one or more biochemical steps that transfer the H in NADH and an extra electron to a molecule of pyruvate. As a result, NADH is restored to NAD+, which is needed for glycolysis, and pyruva ...
Microbial metabolism
Microbial metabolism is the means by which a microbe obtains the energy and nutrients (e.g. carbon) it needs to live and reproduce. Microbes use many different types of metabolic strategies and species can often be differentiated from each other based on metabolic characteristics. The specific metabolic properties of a microbe are the major factors in determining that microbe’s ecological niche, and often allow for that microbe to be useful in industrial processes or responsible for biogeochemical cycles.== Types of microbial metabolism ==All microbial metabolisms can be arranged according to three principles:1. How the organism obtains carbon for synthesising cell mass: autotrophic – carbon is obtained from carbon dioxide (CO2) heterotrophic – carbon is obtained from organic compounds mixotrophic – carbon is obtained from both organic compounds and by fixing carbon dioxide2. How the organism obtains reducing equivalents used either in energy conservation or in biosynthetic reactions: lithotrophic – reducing equivalents are obtained from inorganic compounds organotrophic – reducing equivalents are obtained from organic compounds3. How the organism obtains energy for living and growing: chemotrophic – energy is obtained from external chemical compounds phototrophic – energy is obtained from lightIn practice, these terms are almost freely combined. Typical examples are as follows: chemolithoautotrophs obtain energy from the oxidation of inorganic compounds and carbon from the fixation of carbon dioxide. Examples: Nitrifying bacteria, Sulfur-oxidizing bacteria, Iron-oxidizing bacteria, Knallgas-bacteria photolithoautotrophs obtain energy from light and carbon from the fixation of carbon dioxide, using reducing equivalents from inorganic compounds. Examples: Cyanobacteria (water (H2O) as reducing equivalent donor), Chlorobiaceae, Chromatiaceae (hydrogen sulfide (H2S) as reducing equivalent donor), Chloroflexus (hydrogen (H2) as reducing equivalent donor) chemolithoheterotrophs obtain energy from the oxidation of inorganic compounds, but cannot fix carbon dioxide (CO2). Examples: some Thiobacilus, some Beggiatoa, some Nitrobacter spp., Wolinella (with H2 as reducing equivalent donor), some Knallgas-bacteria, some sulfate-reducing bacteria chemoorganoheterotrophs obtain energy, carbon, and reducing equivalents for biosynthetic reactions from organic compounds. Examples: most bacteria, e. g. Escherichia coli, Bacillus spp., Actinobacteria photoorganoheterotrophs obtain energy from light, carbon and reducing equivalents for biosynthetic reactions from organic compounds. Some species are strictly heterotrophic, many others can also fix carbon dioxide and are mixotrophic. Examples: Rhodobacter, Rhodopseudomonas, Rhodospirillum, Rhodomicrobium, Rhodocyclus, Heliobacterium, Chloroflexus (alternatively to photolithoautotrophy with hydrogen)