How many grams of oxygen are made if 3.75 moles of KClO 3
... 11. The characteristic odor of garlic is due to allyl sulfide (C3H5)2S. A recipe for hummus calls for garlic that contains no more than 6.89 mol of allyl sulfide. You were hired by Cedar as a chemistry consultant to calculate the maximum mass of allyl sulfide that should be included in the recipe fo ...
... 11. The characteristic odor of garlic is due to allyl sulfide (C3H5)2S. A recipe for hummus calls for garlic that contains no more than 6.89 mol of allyl sulfide. You were hired by Cedar as a chemistry consultant to calculate the maximum mass of allyl sulfide that should be included in the recipe fo ...
Ch 9 Cell Respiration HW Packet
... Describe what happens during glycolysis. Describe what happens during the Krebs cycle. Explain how high-energy electrons are used by the electron transport chain. Identify how much ATP cellular respiration generates. Glycolysis - The word glycolysis literally means “sugar-breaking.” The end ...
... Describe what happens during glycolysis. Describe what happens during the Krebs cycle. Explain how high-energy electrons are used by the electron transport chain. Identify how much ATP cellular respiration generates. Glycolysis - The word glycolysis literally means “sugar-breaking.” The end ...
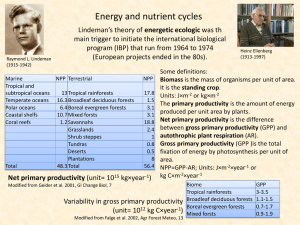
Chapter 6: Energy in the Ecosystem
... organisms living in the same place not only have similar tolerances of physical factors, but feeding relationships link these organisms into a single functional ...
... organisms living in the same place not only have similar tolerances of physical factors, but feeding relationships link these organisms into a single functional ...
The World Within An Ecosystem
... The raw materials needed by plants to produce their own food -with the energy from the Sun - are water and carbon dioxide. The products produced are food (sugars) and oxygen, which is released back into the atmosphere. The food making process is called photosynthesis. The Importance of Photosynthesi ...
... The raw materials needed by plants to produce their own food -with the energy from the Sun - are water and carbon dioxide. The products produced are food (sugars) and oxygen, which is released back into the atmosphere. The food making process is called photosynthesis. The Importance of Photosynthesi ...
Chapter 19
... • a-Ketoglutarate dehydrogenase complex: inhibited by ATP, NADH, and succinyl CoA; activated by ADP and NAD+. ...
... • a-Ketoglutarate dehydrogenase complex: inhibited by ATP, NADH, and succinyl CoA; activated by ADP and NAD+. ...
Organic Chemistry
... 3. Complex carbohydrates (disaccharides and Polysaccharides) can be made from joining together two or more Monosaccharides. This process is called dehydraton syntheisis or condensation reaction. The dehydration part is the removal of water. The synthesis part is the joining of the two smaller compo ...
... 3. Complex carbohydrates (disaccharides and Polysaccharides) can be made from joining together two or more Monosaccharides. This process is called dehydraton syntheisis or condensation reaction. The dehydration part is the removal of water. The synthesis part is the joining of the two smaller compo ...
FREE Sample Here
... 1. Evolution helps scientists decide which technologies can help save the environment. 2. Humans need a renewable resource to produce energy. a) Example: corn or waste products for ethanol production 3. Evolution helps save endangered species as well. 1.10 Evolution from a common ancestor accounts f ...
... 1. Evolution helps scientists decide which technologies can help save the environment. 2. Humans need a renewable resource to produce energy. a) Example: corn or waste products for ethanol production 3. Evolution helps save endangered species as well. 1.10 Evolution from a common ancestor accounts f ...
Lecture 11: Take your Vitamins! Enzyme Cofactors Reference
... -Donates or accepts one proton and two electrons as a hydride ion (H: -) -Commonly used by dehydrogenase enzymes -A true cosubstrate: binds like a substrate and exits like a product. -NADH biggest role? Carry electrons to mitochondria to drive ATP ...
... -Donates or accepts one proton and two electrons as a hydride ion (H: -) -Commonly used by dehydrogenase enzymes -A true cosubstrate: binds like a substrate and exits like a product. -NADH biggest role? Carry electrons to mitochondria to drive ATP ...
Biology, the Study of Life
... 1. Evolution helps scientists decide which technologies can help save the environment. 2. Humans need a renewable resource to produce energy. a) Example: corn or waste products for ethanol production 3. Evolution helps save endangered species as well. 1.10 Evolution from a common ancestor accounts f ...
... 1. Evolution helps scientists decide which technologies can help save the environment. 2. Humans need a renewable resource to produce energy. a) Example: corn or waste products for ethanol production 3. Evolution helps save endangered species as well. 1.10 Evolution from a common ancestor accounts f ...
Respiration
... • Reduced NAD builds up as it is not able to deliver electrons and hydrogen ions to the ETC. • Even glycolysis would stop if no Reduced NAD is reoxidised. • Rather, each pyruvate molecule produced in glycolysis takes hydrogen ions from Reduced NAD – to form Lactic Acid. ...
... • Reduced NAD builds up as it is not able to deliver electrons and hydrogen ions to the ETC. • Even glycolysis would stop if no Reduced NAD is reoxidised. • Rather, each pyruvate molecule produced in glycolysis takes hydrogen ions from Reduced NAD – to form Lactic Acid. ...
Document
... b. Phosphofructokinase, which catalyzes the formation of fructose-1,6-bisphosphate, is inhibited by high levels of ATP, and activated by high levels of ADP and AMP. c. High levels of ATP or acetyl CoA inhibit pyruvate kinase, which stops the formation of pyruvate in reaction 10. ...
... b. Phosphofructokinase, which catalyzes the formation of fructose-1,6-bisphosphate, is inhibited by high levels of ATP, and activated by high levels of ADP and AMP. c. High levels of ATP or acetyl CoA inhibit pyruvate kinase, which stops the formation of pyruvate in reaction 10. ...
electron transport chain.
... • Following glycolysis and the citric acid cycle, NADH and FADH2 account for most of the energy extracted from food. • These two electron carriers donate electrons to the electron transport chain, which powers ATP synthesis via oxidative phosphorylation. ...
... • Following glycolysis and the citric acid cycle, NADH and FADH2 account for most of the energy extracted from food. • These two electron carriers donate electrons to the electron transport chain, which powers ATP synthesis via oxidative phosphorylation. ...
AP Biology - mvhs
... Sap transport in phloem – pressure-flow hypothesis, role of active transport and osmosis in loading at source and unloading at sink Redox reactions – LEO, GER; where do electrons originate, what pulls them away Light- Dependent Reactions – location, purpose; role of chlorophyll, water and photosyste ...
... Sap transport in phloem – pressure-flow hypothesis, role of active transport and osmosis in loading at source and unloading at sink Redox reactions – LEO, GER; where do electrons originate, what pulls them away Light- Dependent Reactions – location, purpose; role of chlorophyll, water and photosyste ...
BIOLOGY 311C - Brand Spring 2007 NAME (printed very legibly
... a. is chemically more reduced. b. is protonated. c. is the D enantiomer of lactate. d. contains a bound molecule of H2O. 17. Which one of the following takes place in the cytoplasmic matrix of eukaryotes? a. The Kreb cycle b. The Calvin Cycle c. Glycolysis d. Transcription 18. During the first few r ...
... a. is chemically more reduced. b. is protonated. c. is the D enantiomer of lactate. d. contains a bound molecule of H2O. 17. Which one of the following takes place in the cytoplasmic matrix of eukaryotes? a. The Kreb cycle b. The Calvin Cycle c. Glycolysis d. Transcription 18. During the first few r ...
1 Glycolysis and carbon-carbon bond chemistry I. Intro to Glycolysis
... the oxidation-reduction state? Two aspects of entropy explain this large free energy ...
... the oxidation-reduction state? Two aspects of entropy explain this large free energy ...
CHEM1405 2003-J-2 June 2003 • Draw the Lewis structure for sulfur
... What shape would that molecule have? Explain. The molecule would be bent with approx 90°° bond angle as the p orbitals are at right angles to each other. What molecule forms instead? Explain. ...
... What shape would that molecule have? Explain. The molecule would be bent with approx 90°° bond angle as the p orbitals are at right angles to each other. What molecule forms instead? Explain. ...
Chapter 10. Photosynthesis: The Calvin Cycle Life
... Need to produce all organic molecules necessary for growth carbohydrates, lipids proteins, nucleic acids ...
... Need to produce all organic molecules necessary for growth carbohydrates, lipids proteins, nucleic acids ...
발효화학-8.
... In microbiology, the term ‘fermentation’ can be used to describe either (1) microbial processes that produce useful products or (2) a form of anaerobic microbial growth using internally supplied electron acceptors and generating ATP mainly through substrate-level phosphorylation (SLP). This chap ...
... In microbiology, the term ‘fermentation’ can be used to describe either (1) microbial processes that produce useful products or (2) a form of anaerobic microbial growth using internally supplied electron acceptors and generating ATP mainly through substrate-level phosphorylation (SLP). This chap ...
File
... needs supply of hydrogen ; to form reduced, NAD / FAD ; lipids have more, hydrogen / hydrogen – carbon bonds ; more acetyl coenzyme A generated / more ‘turns’ of Krebs cycle ; ...
... needs supply of hydrogen ; to form reduced, NAD / FAD ; lipids have more, hydrogen / hydrogen – carbon bonds ; more acetyl coenzyme A generated / more ‘turns’ of Krebs cycle ; ...
Microbial metabolism
Microbial metabolism is the means by which a microbe obtains the energy and nutrients (e.g. carbon) it needs to live and reproduce. Microbes use many different types of metabolic strategies and species can often be differentiated from each other based on metabolic characteristics. The specific metabolic properties of a microbe are the major factors in determining that microbe’s ecological niche, and often allow for that microbe to be useful in industrial processes or responsible for biogeochemical cycles.== Types of microbial metabolism ==All microbial metabolisms can be arranged according to three principles:1. How the organism obtains carbon for synthesising cell mass: autotrophic – carbon is obtained from carbon dioxide (CO2) heterotrophic – carbon is obtained from organic compounds mixotrophic – carbon is obtained from both organic compounds and by fixing carbon dioxide2. How the organism obtains reducing equivalents used either in energy conservation or in biosynthetic reactions: lithotrophic – reducing equivalents are obtained from inorganic compounds organotrophic – reducing equivalents are obtained from organic compounds3. How the organism obtains energy for living and growing: chemotrophic – energy is obtained from external chemical compounds phototrophic – energy is obtained from lightIn practice, these terms are almost freely combined. Typical examples are as follows: chemolithoautotrophs obtain energy from the oxidation of inorganic compounds and carbon from the fixation of carbon dioxide. Examples: Nitrifying bacteria, Sulfur-oxidizing bacteria, Iron-oxidizing bacteria, Knallgas-bacteria photolithoautotrophs obtain energy from light and carbon from the fixation of carbon dioxide, using reducing equivalents from inorganic compounds. Examples: Cyanobacteria (water (H2O) as reducing equivalent donor), Chlorobiaceae, Chromatiaceae (hydrogen sulfide (H2S) as reducing equivalent donor), Chloroflexus (hydrogen (H2) as reducing equivalent donor) chemolithoheterotrophs obtain energy from the oxidation of inorganic compounds, but cannot fix carbon dioxide (CO2). Examples: some Thiobacilus, some Beggiatoa, some Nitrobacter spp., Wolinella (with H2 as reducing equivalent donor), some Knallgas-bacteria, some sulfate-reducing bacteria chemoorganoheterotrophs obtain energy, carbon, and reducing equivalents for biosynthetic reactions from organic compounds. Examples: most bacteria, e. g. Escherichia coli, Bacillus spp., Actinobacteria photoorganoheterotrophs obtain energy from light, carbon and reducing equivalents for biosynthetic reactions from organic compounds. Some species are strictly heterotrophic, many others can also fix carbon dioxide and are mixotrophic. Examples: Rhodobacter, Rhodopseudomonas, Rhodospirillum, Rhodomicrobium, Rhodocyclus, Heliobacterium, Chloroflexus (alternatively to photolithoautotrophy with hydrogen)