DNA-notes
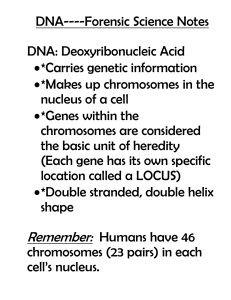
... (Each gene has its own specific location called a LOCUS) *Double stranded, double helix shape ...
... (Each gene has its own specific location called a LOCUS) *Double stranded, double helix shape ...
2770 October 2007 Mid-Term Test
... Alanine and valine are neutral, non-polar amino acids. C) Serine and glutamine are polar, uncharged amino acids. D) Lysine and arginine are basic amino acids. E) Tyrosine and phenylalanine are aromatic amino acids. ...
... Alanine and valine are neutral, non-polar amino acids. C) Serine and glutamine are polar, uncharged amino acids. D) Lysine and arginine are basic amino acids. E) Tyrosine and phenylalanine are aromatic amino acids. ...
Protein Synthesis Poster Project
... Materials: 3 pieces of construction paper, 1 pairs of scissors, crayons, glue or tape, model for tRNAs, construction paper (ribosomes),toothpicks or yarn for peptide bonds. Pre-Activity : BEFORE Beginning Poster: List the following steps of Translation (Protein Synthesis) in order on the back of thi ...
... Materials: 3 pieces of construction paper, 1 pairs of scissors, crayons, glue or tape, model for tRNAs, construction paper (ribosomes),toothpicks or yarn for peptide bonds. Pre-Activity : BEFORE Beginning Poster: List the following steps of Translation (Protein Synthesis) in order on the back of thi ...
Carbon Compounds in Cells
... (ribose or deoxyribose), a nitrogen base, and phosphate group – Adenosine phosphate are chemical messenger (cAMP) or energy carriers (ATP) – Nucleotide coenzyme transport hydrogen atoms and electrons (example NAD+ and FAD) – Nucleotides serve as a building block for nucleic acid ...
... (ribose or deoxyribose), a nitrogen base, and phosphate group – Adenosine phosphate are chemical messenger (cAMP) or energy carriers (ATP) – Nucleotide coenzyme transport hydrogen atoms and electrons (example NAD+ and FAD) – Nucleotides serve as a building block for nucleic acid ...
Notes handout for Basic Biochemistry
... ___________________ bonds with up to 4 other atoms (usually H, O, N, S, P, or another C). Carbon can form long chains, branched structures, or rings. Adjacent carbon atoms can also form Double and Triple bonds. There are four basic classes of organic molecules: carbohydrates, lipids, proteins and nu ...
... ___________________ bonds with up to 4 other atoms (usually H, O, N, S, P, or another C). Carbon can form long chains, branched structures, or rings. Adjacent carbon atoms can also form Double and Triple bonds. There are four basic classes of organic molecules: carbohydrates, lipids, proteins and nu ...
Macromolecules Review ws Name the 6 main elements that make
... 16. Chains of amino acids make polypeptides which can join together to make a protein. 17. Phosholipids makes up cell membranes. 18. Fats are made of an alcohol called glycerol and three fatty acids chains. This is known as a triglyceride 19. If there are all SINGLE bonds between carbons in the fat ...
... 16. Chains of amino acids make polypeptides which can join together to make a protein. 17. Phosholipids makes up cell membranes. 18. Fats are made of an alcohol called glycerol and three fatty acids chains. This is known as a triglyceride 19. If there are all SINGLE bonds between carbons in the fat ...
Chapter 13: RNA and Protein Synthesis
... What is RNA? • RNA (Ribonucleic Acid) – How is RNA physically different from DNA? • 1. Single strand not a double • 2. Contains Ribose and not deoxyribose ...
... What is RNA? • RNA (Ribonucleic Acid) – How is RNA physically different from DNA? • 1. Single strand not a double • 2. Contains Ribose and not deoxyribose ...
Functional Groups, I
... • Lipids: glycerol (m) & fatty acids (m) form lipids (p), steroids (p) and phospholipids (p) • Proteins- amino acids (m) form 4 different conformational proteins (p). • Nucleic acids- nitrogenous base (m), pentose sugar (m) and phosphates (m) make up DNA (p) ...
... • Lipids: glycerol (m) & fatty acids (m) form lipids (p), steroids (p) and phospholipids (p) • Proteins- amino acids (m) form 4 different conformational proteins (p). • Nucleic acids- nitrogenous base (m), pentose sugar (m) and phosphates (m) make up DNA (p) ...
Amino Acid Catabolism
... considered from the origins and fates of their: (1) Nitrogen atoms (2) Carbon skeletons •For mammals: Essential amino acids must be obtained from diet Nonessential amino acids - can be synthesized ...
... considered from the origins and fates of their: (1) Nitrogen atoms (2) Carbon skeletons •For mammals: Essential amino acids must be obtained from diet Nonessential amino acids - can be synthesized ...
The four types of nucleotides in DNA are Adenine, Thymine
... All of the above 2. What does DNA polymerase do? A. Unwinds a strand of DNA so replication can take place B. Creates enzymes used in replication C. Matches the nucleotides on a strand of DNA to their complement D. Generates chemical signals triggering replication 3. Which nucleotide does uracil repl ...
... All of the above 2. What does DNA polymerase do? A. Unwinds a strand of DNA so replication can take place B. Creates enzymes used in replication C. Matches the nucleotides on a strand of DNA to their complement D. Generates chemical signals triggering replication 3. Which nucleotide does uracil repl ...
Translation Tjian lec 26
... synthesis by an aminoacyl-tRNA synthetase enzyme is shown. As indicated, the energy of ATP hydrolysis is used to attach each amino acid to its tRNA molecule in a high-energy linkage. The amino acid is first activated through the linkage of its carboxyl group directly to an AMP moiety, forming and ad ...
... synthesis by an aminoacyl-tRNA synthetase enzyme is shown. As indicated, the energy of ATP hydrolysis is used to attach each amino acid to its tRNA molecule in a high-energy linkage. The amino acid is first activated through the linkage of its carboxyl group directly to an AMP moiety, forming and ad ...
Unit 3: BIOCHEMISTRY REVIEW
... Keep all your review packets to review for semester exam in January. Use your notes and if needed, Chapter 2 in your textbook to complete this. 1. Elements: . What element do these symbols represent? C- ______________ H- ______________ P- ______________ N- ______________ O- ______________ Na- ______ ...
... Keep all your review packets to review for semester exam in January. Use your notes and if needed, Chapter 2 in your textbook to complete this. 1. Elements: . What element do these symbols represent? C- ______________ H- ______________ P- ______________ N- ______________ O- ______________ Na- ______ ...
Match each macromolecule (Carbohydrates, Proteins, Lipids
... the cell membrane, other examples include vitamins, cholesterol, estrogen, and testosterone. _________________________________ ...
... the cell membrane, other examples include vitamins, cholesterol, estrogen, and testosterone. _________________________________ ...
Distinguish between these 3 root types: - mvhs
... a nucleic acid 3’ - closest to carbon 3 of the sugar in a nucleic acid Label the carbons in the nucleotide below: ...
... a nucleic acid 3’ - closest to carbon 3 of the sugar in a nucleic acid Label the carbons in the nucleotide below: ...
UTACCEL 2010
... DNA is a long double-stranded molecule residing inside the nucleus of every cell. It is usually tightly coiled forming chromosomes in which it is protected by proteins. ...
... DNA is a long double-stranded molecule residing inside the nucleus of every cell. It is usually tightly coiled forming chromosomes in which it is protected by proteins. ...
1018-1635_Chan
... Introduction: Carbonaceous Chondrites (CC) are primitive meteorites that have not experienced extensive planetary differentiation. They also contain carbon up to 5% by weight, most of which is organic in nature. It is speculated that these extraterrestrial materials might have contributed to a sourc ...
... Introduction: Carbonaceous Chondrites (CC) are primitive meteorites that have not experienced extensive planetary differentiation. They also contain carbon up to 5% by weight, most of which is organic in nature. It is speculated that these extraterrestrial materials might have contributed to a sourc ...
Chapter 6, Section 3
... carbon atoms that are covalently bonded to other carbon atoms and other elements such as oxygen, hydrogen, and nitrogen. 1. Carbon forms bonds easily because it has 4 valence electrons. 2. Carbon atoms can bond to other carbon atoms, forming chains that are almost unlimited in length. 3. All living ...
... carbon atoms that are covalently bonded to other carbon atoms and other elements such as oxygen, hydrogen, and nitrogen. 1. Carbon forms bonds easily because it has 4 valence electrons. 2. Carbon atoms can bond to other carbon atoms, forming chains that are almost unlimited in length. 3. All living ...
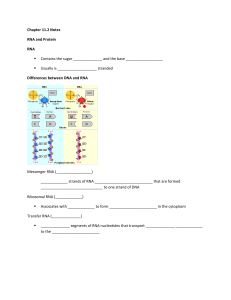
Chapter 11.2 Notes RNA and Protein RNA Contains the sugar and
... ____________________ – the process of ________________________ the info in a sequence of nitrogenous ______________ in mRNA into a sequence of amino acids in _______________ ...
... ____________________ – the process of ________________________ the info in a sequence of nitrogenous ______________ in mRNA into a sequence of amino acids in _______________ ...
Unit 1 objectives and vocab
... Carbohydrate Monosaccharide Fatty Acid Polysaccharide Lipid Nucleic acid Nucleotide Protein Biomolecule Calorie ...
... Carbohydrate Monosaccharide Fatty Acid Polysaccharide Lipid Nucleic acid Nucleotide Protein Biomolecule Calorie ...
Biosynthesis

Biosynthesis (also called biogenesis or anabolism) is a multi-step, enzyme-catalyzed process where substrates are converted into more complex products in living organisms. In biosynthesis, simple compounds are modified, converted into other compounds, or joined together to form macromolecules. This process often consists of metabolic pathways. Some of these biosynthetic pathways are located within a single cellular organelle, while others involve enzymes that are located within multiple cellular organelles. Examples of these biosynthetic pathways include the production of lipid membrane components and nucleotides.The prerequisite elements for biosynthesis include: precursor compounds, chemical energy (e.g. ATP), and catalytic enzymes which may require coenzymes (e.g.NADH, NADPH). These elements create monomers, the building blocks for macromolecules. Some important biological macromolecules include: proteins, which are composed of amino acid monomers joined via peptide bonds, and DNA molecules, which are composed of nucleotides joined via phosphodiester bonds.