14 more about the atomic number
... Each element in the Periodic Table has its own atomic number. The atomic number is important it gives the number of protons in an atom of an element. Since atoms are neutral and the charge on an electron is equal and opposite to the charge on a proton, the atomic number also gives the number of elec ...
... Each element in the Periodic Table has its own atomic number. The atomic number is important it gives the number of protons in an atom of an element. Since atoms are neutral and the charge on an electron is equal and opposite to the charge on a proton, the atomic number also gives the number of elec ...
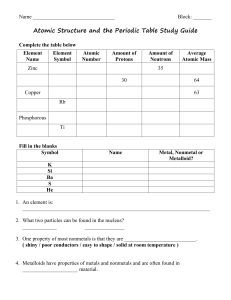
VOCABULARY name, date, hour: Fill in the number of each term
... ___ substance that is a mixture of two or more metals ___ columns of the periodic table; also known as groups ___ number of protons carried by the nucleus of an atom ___ element with an imbalance in the number of neutrons and protons ___ uncharged particle found in the nucleus of an atom ___ physica ...
... ___ substance that is a mixture of two or more metals ___ columns of the periodic table; also known as groups ___ number of protons carried by the nucleus of an atom ___ element with an imbalance in the number of neutrons and protons ___ uncharged particle found in the nucleus of an atom ___ physica ...
Chapter 2
... 4. Draw and label a simplified model of an atom. Explain how this model simplifies our understanding of atomic structure. 5. Distinguish between each of the following pairs of terms: a. neutron and proton b. atomic number and mass number c. atomic weight and mass number 6. Explain how the atomic num ...
... 4. Draw and label a simplified model of an atom. Explain how this model simplifies our understanding of atomic structure. 5. Distinguish between each of the following pairs of terms: a. neutron and proton b. atomic number and mass number c. atomic weight and mass number 6. Explain how the atomic num ...
ChLM Final Review Name: Period: Base Knowledge 1. Classify the
... 37. Draw the complete Bohr model for Beryllium and Magnesium (remember to show protons & neutrons). What do the electrons have in common between these two elements? ...
... 37. Draw the complete Bohr model for Beryllium and Magnesium (remember to show protons & neutrons). What do the electrons have in common between these two elements? ...
ch2_objectives
... 9. Define the terms energy and potential energy. Explain why electrons in the first electron shell have less potential energy than electrons in higher electron shells. 10. Distinguish among nonpolar covalent, polar covalent and ionic bonds. 11. Explain why strong covalent bonds and weak bonds are b ...
... 9. Define the terms energy and potential energy. Explain why electrons in the first electron shell have less potential energy than electrons in higher electron shells. 10. Distinguish among nonpolar covalent, polar covalent and ionic bonds. 11. Explain why strong covalent bonds and weak bonds are b ...
Chemistry10AtomicTheory
... Elements are made of tiny particles called atoms. All atoms of a given element are identical. The atoms of a given element are different from those of any ...
... Elements are made of tiny particles called atoms. All atoms of a given element are identical. The atoms of a given element are different from those of any ...
Isotopes
... 1. Use Video #5 – to take notes over isotopes on the back of your paper from today. Then write the paragraph at the bottom of the page. 2. Study for quiz tomorrow! (over scientists and subatomic particles) REMINDERS ...
... 1. Use Video #5 – to take notes over isotopes on the back of your paper from today. Then write the paragraph at the bottom of the page. 2. Study for quiz tomorrow! (over scientists and subatomic particles) REMINDERS ...
Topic 4: Classifying Elements What did the early chemists use to
... • NH3(g) à nitrogen trihydride or ammonia • CH4(g) à carbon tetrahydride or methane • H2O2(l) à dihydrogen monoxide or water A MOLECULAR COMPOUND can contain what two combinations of elements? Non-‐metal + ...
... • NH3(g) à nitrogen trihydride or ammonia • CH4(g) à carbon tetrahydride or methane • H2O2(l) à dihydrogen monoxide or water A MOLECULAR COMPOUND can contain what two combinations of elements? Non-‐metal + ...
+ 2 HCL(aq) CaCl2(aq) + H2O(l) + CO2(g)
... Subscript: A number that represents how many atoms of an element are in a compound. Compound: A substance made of the combined atoms of two or more elements. Chemical Formula: States what elements a compound contains and the exact number of atoms of these elements. Oxidation Number: positive or nega ...
... Subscript: A number that represents how many atoms of an element are in a compound. Compound: A substance made of the combined atoms of two or more elements. Chemical Formula: States what elements a compound contains and the exact number of atoms of these elements. Oxidation Number: positive or nega ...
and View
... atom. * Number never changes* b. Isotopes—atoms of same element that have different numbers of neutrons. Ex: carbon-12, carbon-13, carbon-14 c. Mass number—number of neutrons plus protons in an atom. i. Neutron number is found by--Mass number - Atomic number _______________ Number of neutrons ...
... atom. * Number never changes* b. Isotopes—atoms of same element that have different numbers of neutrons. Ex: carbon-12, carbon-13, carbon-14 c. Mass number—number of neutrons plus protons in an atom. i. Neutron number is found by--Mass number - Atomic number _______________ Number of neutrons ...
Early Chemistry Development of the Atomic Model
... For the next 2000 years no major developments but, experimentation is done by alchemists. The beginning of modern chemical experimentation comes in 1661 when Robert Boyle publishes "The Skeptical Chymist". Boyle studies primarily the behavior of gasses and introduces the concept of elements. The fir ...
... For the next 2000 years no major developments but, experimentation is done by alchemists. The beginning of modern chemical experimentation comes in 1661 when Robert Boyle publishes "The Skeptical Chymist". Boyle studies primarily the behavior of gasses and introduces the concept of elements. The fir ...
Extra Credit Test Review
... 12.One atom has 17 protons, 18 neutrons, and 17 electrons. Another atom has 17 protons, 19 neutrons and 17 electrons. Are these the same element? Yes No Explain: __________________________________________________________________ 13.Today we use Mendeleev’s arrangement, elements are arranged by incre ...
... 12.One atom has 17 protons, 18 neutrons, and 17 electrons. Another atom has 17 protons, 19 neutrons and 17 electrons. Are these the same element? Yes No Explain: __________________________________________________________________ 13.Today we use Mendeleev’s arrangement, elements are arranged by incre ...
Elements and Atoms - Portola Middle School
... building blocks of all matter. • The periodic table is a list of all of the elements that can build matter. It’s a little like the alphabet of chemistry. • The periodic table tells us several things… ...
... building blocks of all matter. • The periodic table is a list of all of the elements that can build matter. It’s a little like the alphabet of chemistry. • The periodic table tells us several things… ...
answers
... c.) Rutherford – discovered positively charged nucleus d.) Bohr – solar system model of atoms, energy levels at increasing distance from nucleus ...
... c.) Rutherford – discovered positively charged nucleus d.) Bohr – solar system model of atoms, energy levels at increasing distance from nucleus ...
Name Test Review Chemistry Unit 2: The Atom 1. Fill in the blank
... 7. What is the mass of 0.44 moles of carbon? 8. How many atoms does 43.25 g of iron contain? 9. If a student weighs out 2.01 g of silicon, how many moles is that? 10. How many moles are there in 2.4010 x 1025 particles of gold? 11. What is the mass of a block of aluminum that has a volume of 22.4 cm ...
... 7. What is the mass of 0.44 moles of carbon? 8. How many atoms does 43.25 g of iron contain? 9. If a student weighs out 2.01 g of silicon, how many moles is that? 10. How many moles are there in 2.4010 x 1025 particles of gold? 11. What is the mass of a block of aluminum that has a volume of 22.4 cm ...
Reading Comprehension - Easy Peasy All-in
... atoms that are all the same kind. It is a pure form of matter. Elements join together with other elements to make the different materials that we see and use every day. Some common elements that you might have heard about are oxygen, carbon, helium, gold and silver. If you have a lump of silver, all ...
... atoms that are all the same kind. It is a pure form of matter. Elements join together with other elements to make the different materials that we see and use every day. Some common elements that you might have heard about are oxygen, carbon, helium, gold and silver. If you have a lump of silver, all ...
Ch 3 studentElements Ions Isotopes
... 2. all atoms of a particular element are identical 3. different elements have different atoms 4. atoms combine in certain whole-number ratios 5. In a chemical reaction, atoms are merely rearranged to form new compounds; they are not created, destroyed, or changed into atoms of any other elements ...
... 2. all atoms of a particular element are identical 3. different elements have different atoms 4. atoms combine in certain whole-number ratios 5. In a chemical reaction, atoms are merely rearranged to form new compounds; they are not created, destroyed, or changed into atoms of any other elements ...
Chapter 3 Atoms and Elements
... All light, whether radiowaves or visible light, travels as the same speed, 3 *108 meters/sec As a result, since the length of each wave decreases from left to right, the frequency of the peaks an troughs of the waves shown above must increase from left to right Referring to light as a particle, know ...
... All light, whether radiowaves or visible light, travels as the same speed, 3 *108 meters/sec As a result, since the length of each wave decreases from left to right, the frequency of the peaks an troughs of the waves shown above must increase from left to right Referring to light as a particle, know ...
Atomic Structure Power Point
... Hydrogen has 1 proton, so its atomic no. is 1. Hydrogen is element #1 on the Periodic Table of the Elements. ...
... Hydrogen has 1 proton, so its atomic no. is 1. Hydrogen is element #1 on the Periodic Table of the Elements. ...
Honors Review Unit 2 answers
... 22. If the half-life for the radioactive decay of zirconium-84 is 26 minutes and I start with a 175 gram sample, how much will be left over after 104 minutes? 10.9 g 23. Mercury -197 is used for kidney scans and has a half-life of 3 days. If the amount of mercury-197 needed for a study is 1.0 gram ...
... 22. If the half-life for the radioactive decay of zirconium-84 is 26 minutes and I start with a 175 gram sample, how much will be left over after 104 minutes? 10.9 g 23. Mercury -197 is used for kidney scans and has a half-life of 3 days. If the amount of mercury-197 needed for a study is 1.0 gram ...
Name Test Review Chapters 4 and 25 Honors Chemistry 1. Fill in
... 11. What is the mass of a block of aluminum that has a volume of 22.4 cm3? (Density of Al = 2.70 g/cm3) 12. If a metal cylinder of copper (density = 8.9 g/cm3) has a mass of 45.4 grams, what is the volume of the cylinder? What is the cylinder’s diameter if it is 1.2 cm in height? 13. Convert 2950 m ...
... 11. What is the mass of a block of aluminum that has a volume of 22.4 cm3? (Density of Al = 2.70 g/cm3) 12. If a metal cylinder of copper (density = 8.9 g/cm3) has a mass of 45.4 grams, what is the volume of the cylinder? What is the cylinder’s diameter if it is 1.2 cm in height? 13. Convert 2950 m ...