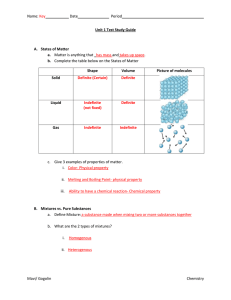
File
... Matter is anything that has mass and takes up space. Mass is the amount of matter (how much stuff) an object contains. The mass of an object will not change if the force of gravity on it changes. For mass, the SI unit is kilogram (kg). The amount of space that matter occupies is the volume. Common u ...
... Matter is anything that has mass and takes up space. Mass is the amount of matter (how much stuff) an object contains. The mass of an object will not change if the force of gravity on it changes. For mass, the SI unit is kilogram (kg). The amount of space that matter occupies is the volume. Common u ...
atomic structure - IGCSE STUDY BANK
... Isotopes are atoms of the same element with different numbers of neutrons. This gives each isotope of the element a different mass or nucleon number but being the same element they have the same atomic or proton number. There are small physical differences between the isotopes eg the heavier isotope ...
... Isotopes are atoms of the same element with different numbers of neutrons. This gives each isotope of the element a different mass or nucleon number but being the same element they have the same atomic or proton number. There are small physical differences between the isotopes eg the heavier isotope ...
World of
... 3.Atoms of a given element are different from those of any other element. 4.Atoms of one can combine with atoms of another to form compounds ...
... 3.Atoms of a given element are different from those of any other element. 4.Atoms of one can combine with atoms of another to form compounds ...
Atomic Structure
... • If an atom were the size of a football stadium, the nucleus would be about the size of a marble. ...
... • If an atom were the size of a football stadium, the nucleus would be about the size of a marble. ...
Chapter 18
... that comprise protons and neutrons • Scientists have confirmed the existence of six uniquely different quarks • The search for the composition of protons and neutrons is an ongoing ...
... that comprise protons and neutrons • Scientists have confirmed the existence of six uniquely different quarks • The search for the composition of protons and neutrons is an ongoing ...
Name Objective 1: Matter and Energy C3H8 + 5O2 → 3CO2 + 4H2O
... a. C3H8 and C2H6 b. NO2 and KCl c. 2Li2S and Be4Cl2 d. 2CO and CO2 17. All of the following are indicators of a chemical change except — (8.5E) a. formation of a gas b. change in temperature c. change in the state of matter d. formation of a precipitate 18. Why is the compound CaH10P4K3O4 an inorgan ...
... a. C3H8 and C2H6 b. NO2 and KCl c. 2Li2S and Be4Cl2 d. 2CO and CO2 17. All of the following are indicators of a chemical change except — (8.5E) a. formation of a gas b. change in temperature c. change in the state of matter d. formation of a precipitate 18. Why is the compound CaH10P4K3O4 an inorgan ...
File
... TUESDAY: Atoms, Elements, Compounds, & Mixtures (worth 33 percent) True or False: Identify if the following statements are true or false. 1. __________________: Atoms are smallest form of matter. 2. __________________: Electrons are located in the nucleus of an atom. 3. __________________: When two ...
... TUESDAY: Atoms, Elements, Compounds, & Mixtures (worth 33 percent) True or False: Identify if the following statements are true or false. 1. __________________: Atoms are smallest form of matter. 2. __________________: Electrons are located in the nucleus of an atom. 3. __________________: When two ...
Vocabulary for Periodic Table
... 7) Isotope: atoms of the same element that have a different number of neutrons. 8) Ion: when an atom loses or gains electrons. 9) Atomic mass: the average mass of the atoms in an element. 10) Periodic Table: a table of elements, arranged by atomic number, that shows the patterns in their properties. ...
... 7) Isotope: atoms of the same element that have a different number of neutrons. 8) Ion: when an atom loses or gains electrons. 9) Atomic mass: the average mass of the atoms in an element. 10) Periodic Table: a table of elements, arranged by atomic number, that shows the patterns in their properties. ...
Study Guide Answer Key
... Gold Foil Experiment- Rutherford designed an experiment to use the alpha particles emitted by a radioactive element as probes to the unseen world of atomic structure. They shot alpha particles at a sheet of gold foil, and noticed that most went through, but some bounced back. ...
... Gold Foil Experiment- Rutherford designed an experiment to use the alpha particles emitted by a radioactive element as probes to the unseen world of atomic structure. They shot alpha particles at a sheet of gold foil, and noticed that most went through, but some bounced back. ...
ISOTOPES 3 SUBATOMIC PARTICLES Proton Located inside the
... Located outside of the nucleus in an “electron cloud” Involved in chemical bonding Negative charge Equal to the # of protons in a neutral atom How many electrons does Potassium have? How many electrons does Nitrogen have? o Neutron Located inside the nucleus of an atom No charge # ...
... Located outside of the nucleus in an “electron cloud” Involved in chemical bonding Negative charge Equal to the # of protons in a neutral atom How many electrons does Potassium have? How many electrons does Nitrogen have? o Neutron Located inside the nucleus of an atom No charge # ...
Slide 1
... P. 124 – Q – 76 Rutherford’s atomic theory proposed a dense nucleus surrounded by very small electrons. This implies that atoms are composed mainly of empty space. If all matter is mainly empty space, why is it impossible to walk through walls or pass your hand through your desk? P. 122 – Q – 46 Wh ...
... P. 124 – Q – 76 Rutherford’s atomic theory proposed a dense nucleus surrounded by very small electrons. This implies that atoms are composed mainly of empty space. If all matter is mainly empty space, why is it impossible to walk through walls or pass your hand through your desk? P. 122 – Q – 46 Wh ...
Isotopes
... Know how to calculate the number of protons, electrons, and neutrons. I’m going to ask you to complete a table like on our ...
... Know how to calculate the number of protons, electrons, and neutrons. I’m going to ask you to complete a table like on our ...
The_Atoms_Family
... The number and arrangement of electrons determines the size of the atom Electrons move around the nucleus in energy levels and each can only hold so many electrons 1. 1st: 2 electrons 2. 2nd: 8 electrons 3. 3rd: 18 electrons 4. 4th: 32 electrons The number of electrons in the outermost energy level, ...
... The number and arrangement of electrons determines the size of the atom Electrons move around the nucleus in energy levels and each can only hold so many electrons 1. 1st: 2 electrons 2. 2nd: 8 electrons 3. 3rd: 18 electrons 4. 4th: 32 electrons The number of electrons in the outermost energy level, ...
Exam III Review
... 5. An Iron atom is 1.72 angstroms (Å) wide. How many iron atoms would you have to stack end to end to stretch the width of this piece of paper, 8.5 inches? (remember: 1 Å = 10-10 m and 1 inch = 2.54 cm) ...
... 5. An Iron atom is 1.72 angstroms (Å) wide. How many iron atoms would you have to stack end to end to stretch the width of this piece of paper, 8.5 inches? (remember: 1 Å = 10-10 m and 1 inch = 2.54 cm) ...
Chemistry Semester One Exam Review Name:
... 11. Write the electron configurations for the following elements. LithiumNitrogenZincBromineBarium12. What is the characteristic set of valence electrons for the following groups on the periodic table? Alkali metals (1); alkaline earth metals (2); halogens (17); noble gases (18) ...
... 11. Write the electron configurations for the following elements. LithiumNitrogenZincBromineBarium12. What is the characteristic set of valence electrons for the following groups on the periodic table? Alkali metals (1); alkaline earth metals (2); halogens (17); noble gases (18) ...
Lecture 2 - U of L Class Index
... An element is defined by its atomic number. Changing the number of protons in an atom (as in a nuclear reaction) changes the element. While atoms of the same element must have the same atomic number, they may have different mass numbers. If so, they are referred to as isotopes. Most elements have mo ...
... An element is defined by its atomic number. Changing the number of protons in an atom (as in a nuclear reaction) changes the element. While atoms of the same element must have the same atomic number, they may have different mass numbers. If so, they are referred to as isotopes. Most elements have mo ...
Lecture 2
... Is there a simple reaction which only one isotope undergoes? What is the main difference between isotopes of an element? How can we take advantage of that difference? ...
... Is there a simple reaction which only one isotope undergoes? What is the main difference between isotopes of an element? How can we take advantage of that difference? ...
BC1 Atoms Unit Standards
... of the protons, neutrons, and electrons in an atom of an element. 2a For a given element, determine the number of protons 2b When given a number of protons, identify the element name and symbol 2c Identify the number of neutrons in an atom from atomic number and mass number 2d Identify the number of ...
... of the protons, neutrons, and electrons in an atom of an element. 2a For a given element, determine the number of protons 2b When given a number of protons, identify the element name and symbol 2c Identify the number of neutrons in an atom from atomic number and mass number 2d Identify the number of ...
Atomic Timeline
... Isotopes – atoms of an element that have the same number of protons but different numbers of neutrons. This change in neutrons is reflected in the Mass Number ...
... Isotopes – atoms of an element that have the same number of protons but different numbers of neutrons. This change in neutrons is reflected in the Mass Number ...
Test Review: Unit 1 - Ms. Hill`s Pre
... a. Law of conservation of Mass: Mass is neither created nor destroyed during normal chemical and physical changes (Dalton didn’t describe/clarify normal circumstances, matter can be created and destroyed in nuclear reactions) b. Law of Definite Proportions: the fact that a chemical compound contain ...
... a. Law of conservation of Mass: Mass is neither created nor destroyed during normal chemical and physical changes (Dalton didn’t describe/clarify normal circumstances, matter can be created and destroyed in nuclear reactions) b. Law of Definite Proportions: the fact that a chemical compound contain ...
Periodic Table Quiz
... They have the ability to be pulled into wires. They have the ability to be hammered into sheets. They have the ability to be liquid at room temperature. They have the ability to conduct electricity only under certain conditions. ...
... They have the ability to be pulled into wires. They have the ability to be hammered into sheets. They have the ability to be liquid at room temperature. They have the ability to conduct electricity only under certain conditions. ...
Atomic Number, Mass Number, and Isotopes
... IONS: Just as isotopes have different numbers of neutrons, ions have different numbers of electrons. Because protons are positively charged, and electrons are negatively charged, atoms are considered neutral because the numbers of each are equal. However, ions have different numbers of protons and e ...
... IONS: Just as isotopes have different numbers of neutrons, ions have different numbers of electrons. Because protons are positively charged, and electrons are negatively charged, atoms are considered neutral because the numbers of each are equal. However, ions have different numbers of protons and e ...