Lesson Outline - WordPress.com
... up 80.22%. What is the average atomic mass of Boron? 2. Silver has two isotopes, the first has a mass of 106.9 u and an abundance of 51.8%, the second has a mass of 108.9 u and an abundance of 48.2. What is the average atomic mass of ...
... up 80.22%. What is the average atomic mass of Boron? 2. Silver has two isotopes, the first has a mass of 106.9 u and an abundance of 51.8%, the second has a mass of 108.9 u and an abundance of 48.2. What is the average atomic mass of ...
Lecture 2
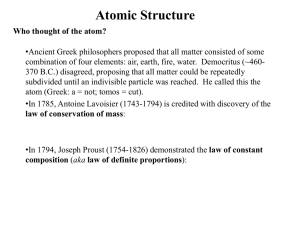
... Who thought of the atom? •Ancient Greek philosophers proposed that all matter consisted of some combination of four elements: air, earth, fire, water. Democritus (~460370 B.C.) disagreed, proposing that all matter could be repeatedly subdivided until an indivisible particle was reached. He called th ...
... Who thought of the atom? •Ancient Greek philosophers proposed that all matter consisted of some combination of four elements: air, earth, fire, water. Democritus (~460370 B.C.) disagreed, proposing that all matter could be repeatedly subdivided until an indivisible particle was reached. He called th ...
AP Chem Test 5-7 Practice Exam - mvhs
... 2. The table above shows the first three ionization energies for atoms of four elements from the third period of the periodic table. The elements are numbered randomly. Use the information in the table to answer the following questions. (a) Which element is most metallic in character? Explain your r ...
... 2. The table above shows the first three ionization energies for atoms of four elements from the third period of the periodic table. The elements are numbered randomly. Use the information in the table to answer the following questions. (a) Which element is most metallic in character? Explain your r ...
File
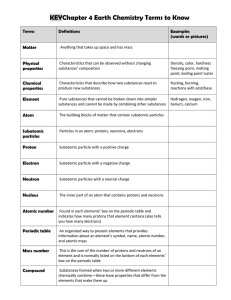
... Found in each elements’ box on the periodic table and indicates how many protons that element contains (also tells you how many electrons) ...
... Found in each elements’ box on the periodic table and indicates how many protons that element contains (also tells you how many electrons) ...
Chemistry lecture notes
... only one stable isotope (e.g. 19F, 27Al, 31P). others may have several (e.g. 1H and 2H, the latter also being called deuterium, 12C and 13C). Molar mass is also known as Relative atomic mass (RAM) is determine by the Proportions (Mixture of the isotopes of an element). ...
... only one stable isotope (e.g. 19F, 27Al, 31P). others may have several (e.g. 1H and 2H, the latter also being called deuterium, 12C and 13C). Molar mass is also known as Relative atomic mass (RAM) is determine by the Proportions (Mixture of the isotopes of an element). ...
2.9 Use the helium-4 isotope to define atomic number and mass
... 2.23 Elements whose names end with ium are usually metals; sodium is one example. Identify a nonmetal whose name also ends with ium. ...
... 2.23 Elements whose names end with ium are usually metals; sodium is one example. Identify a nonmetal whose name also ends with ium. ...
Metals
... “elements”: air, fire, water, and earth. People believed this for many centuries! • In the late 1600s, early chemists began to discover that this was not the case, that there are more than 4 elements and they are not what the Greeks thought they were. • Now we know that all matter in the universe is ...
... “elements”: air, fire, water, and earth. People believed this for many centuries! • In the late 1600s, early chemists began to discover that this was not the case, that there are more than 4 elements and they are not what the Greeks thought they were. • Now we know that all matter in the universe is ...
I can describe an atom and its components I can relate energy levels
... ○ All atoms of the same element have the same number of protons ○ The number of neutrons can vary ○ ex)Chlorine atoms have 17 protons but can have 18 or 20 neutrons. ■ There are chlorine atoms with mass #s of 35 and 37. (17+18=35, 17+20=37) ...
... ○ All atoms of the same element have the same number of protons ○ The number of neutrons can vary ○ ex)Chlorine atoms have 17 protons but can have 18 or 20 neutrons. ■ There are chlorine atoms with mass #s of 35 and 37. (17+18=35, 17+20=37) ...
Isotopes and Ions - Wando High School
... Ions IONS are charged atoms (or groups of atoms) that have a ...
... Ions IONS are charged atoms (or groups of atoms) that have a ...
AlBr3 E IO Ionic FU C O Cov Molec C IO Cov Molec Sn E N/A N/A
... The main characteristic of an element is the atomic number. Two elements differ from each other by their atomic numbers. In fact, atoms of two different elements may have masses that are very close to each other. For example, 39.9624 amu for an atom of argon-40 and 39.9640 amu for an atom of potassi ...
... The main characteristic of an element is the atomic number. Two elements differ from each other by their atomic numbers. In fact, atoms of two different elements may have masses that are very close to each other. For example, 39.9624 amu for an atom of argon-40 and 39.9640 amu for an atom of potassi ...
Atomic Structure AKS Correlation Use the modern atomic theory to
... formed by 2 or more atoms Describe the basic structure of the atom as protons, neutrons and electrons in specific arrangements. Identify the relative location, size and charge of subatomic particles. Define atom. What charge does an atom have? Fill in chart below proton neutron electron Location Nuc ...
... formed by 2 or more atoms Describe the basic structure of the atom as protons, neutrons and electrons in specific arrangements. Identify the relative location, size and charge of subatomic particles. Define atom. What charge does an atom have? Fill in chart below proton neutron electron Location Nuc ...
Section 1 Review
... 5. Infer Sodium and potassium are in the same group on the periodic table. Name ...
... 5. Infer Sodium and potassium are in the same group on the periodic table. Name ...
Lecture 3
... Since in a “handful” of Cl there is a mixture of two isotopes in the abundances shown on the left, an average atomic mass has been defined Average Atomic Mass Cl = 0.7576(Cl35) + 0.2434(Cl37) ...
... Since in a “handful” of Cl there is a mixture of two isotopes in the abundances shown on the left, an average atomic mass has been defined Average Atomic Mass Cl = 0.7576(Cl35) + 0.2434(Cl37) ...
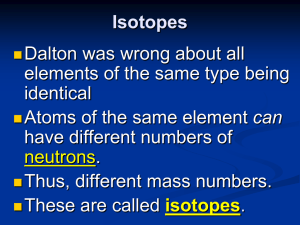
Isotopes
... Isotopes are atoms of the same element having different masses, due to varying numbers of neutrons. ...
... Isotopes are atoms of the same element having different masses, due to varying numbers of neutrons. ...
CHAPTER 3 sec 1 - Leon County Schools
... Can you still see it? NO? Of course not, it’s been reduced to the size of an ...
... Can you still see it? NO? Of course not, it’s been reduced to the size of an ...
8.P.1.1 Warm-Up Questions for Website
... is made up of one type of atom. B.It can be formed through a physical reaction. C.It can be changed into simpler substances through a physical change. D.It is a pure substance containing elements that are chemically combined. ...
... is made up of one type of atom. B.It can be formed through a physical reaction. C.It can be changed into simpler substances through a physical change. D.It is a pure substance containing elements that are chemically combined. ...
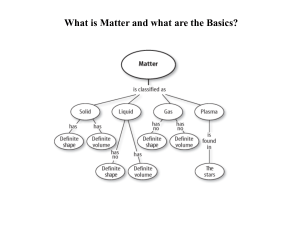
Matter and the Periodic Table
... system of rows and columns on the basis of increasing mass and similar chemical and physical properties. Since the organization exhibited a periodic repetition of similar properties, it became known as the Periodic Table of the Elements. It has become one of modern chemistry's ...
... system of rows and columns on the basis of increasing mass and similar chemical and physical properties. Since the organization exhibited a periodic repetition of similar properties, it became known as the Periodic Table of the Elements. It has become one of modern chemistry's ...