Atoms and Molecules video guide
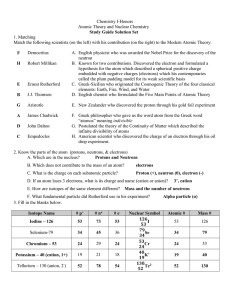
... 13. What does an element’s atomic mass tell about the atoms that make up that ...
... 13. What does an element’s atomic mass tell about the atoms that make up that ...
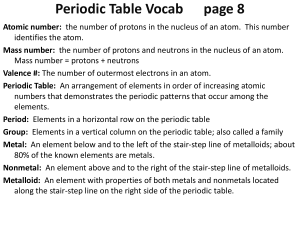
Periodic Table Vocab page 7
... Mass number: the number of protons and neutrons in the nucleus of an atom. Mass number = protons + neutrons Valence #: The number of outermost electrons in an atom. Periodic Table: An arrangement of elements in order of increasing atomic numbers that demonstrates the periodic patterns that occur amo ...
... Mass number: the number of protons and neutrons in the nucleus of an atom. Mass number = protons + neutrons Valence #: The number of outermost electrons in an atom. Periodic Table: An arrangement of elements in order of increasing atomic numbers that demonstrates the periodic patterns that occur amo ...
Exam 1 Review Questions
... All of the elements that are naturally occurring can be found in their pure form in nature. All of the halogens are gases, and don't react with other elements to form compounds. Alloys have a set ratio of elements. Aluminum is in the 4th period in the Periodic Table. Argon, xenon, krypton, and neon ...
... All of the elements that are naturally occurring can be found in their pure form in nature. All of the halogens are gases, and don't react with other elements to form compounds. Alloys have a set ratio of elements. Aluminum is in the 4th period in the Periodic Table. Argon, xenon, krypton, and neon ...
Page 233 - ClassZone
... the world differed because each was made of atoms of different sizes and shapes. How does the modern view of atoms differ from this ancient view? How is it similar? ...
... the world differed because each was made of atoms of different sizes and shapes. How does the modern view of atoms differ from this ancient view? How is it similar? ...
Periodic Table
... Calculating Atomic Mass • Copper exists as a mixture of two isotopes.The lighter isotope (Cu-63), with 29 protons and 34 neutrons, makes up 69.17% of copper atoms.The heavier isotope (Cu-65), with 29 protons and 36 neutrons, constitutes the remaining 30.83% of copper atoms. Calculate the atomic mas ...
... Calculating Atomic Mass • Copper exists as a mixture of two isotopes.The lighter isotope (Cu-63), with 29 protons and 34 neutrons, makes up 69.17% of copper atoms.The heavier isotope (Cu-65), with 29 protons and 36 neutrons, constitutes the remaining 30.83% of copper atoms. Calculate the atomic mas ...
MrsB-Chemistry
... B. A scientist thought that matter could not be divided into smaller pieces because chemical reactions only combine elements. They don’t cause elements to change into other elements. C. Alpha particles were used like bullets, and small, positively charged particles shot out from the center of the at ...
... B. A scientist thought that matter could not be divided into smaller pieces because chemical reactions only combine elements. They don’t cause elements to change into other elements. C. Alpha particles were used like bullets, and small, positively charged particles shot out from the center of the at ...
Quiz: The Atom (Open Notes)
... 10. T or F A neutron carries a positive charge. 11. T or F A proton has a charge that is equal in force but opposite in charge to each electron. 12. T or F Protons and electrons are about equal in mass. 13. T or F The mass of an atom depends on the number of protons and neutrons in its nucleus. 14. ...
... 10. T or F A neutron carries a positive charge. 11. T or F A proton has a charge that is equal in force but opposite in charge to each electron. 12. T or F Protons and electrons are about equal in mass. 13. T or F The mass of an atom depends on the number of protons and neutrons in its nucleus. 14. ...
Intro to Atoms Clicker Questions 1. "atomos" means? 2. Atoms of one
... 2. Atoms of one kind of element _______ be changed into a different element with ordinary chemical means. (can, can’t) 3. Every compound is composed of atoms of different elements combined how? 4. In Thompson's model of the atom, the negatively charged electrons were located how in the atom? 5. In R ...
... 2. Atoms of one kind of element _______ be changed into a different element with ordinary chemical means. (can, can’t) 3. Every compound is composed of atoms of different elements combined how? 4. In Thompson's model of the atom, the negatively charged electrons were located how in the atom? 5. In R ...
Elements Unit Test
... Directions: In your own words, describe the make up of an atom. Be sure to include all proper names of each of its parts. You will be marked out of 5 for your ability to answer the question using complete sentences and providing specific examples or information. ...
... Directions: In your own words, describe the make up of an atom. Be sure to include all proper names of each of its parts. You will be marked out of 5 for your ability to answer the question using complete sentences and providing specific examples or information. ...
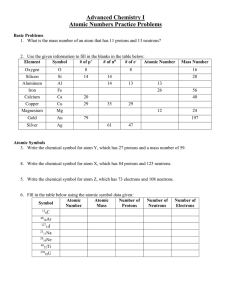
Atomic Numbers Practice Problems
... 7. With respect to the three fundamental particles of atoms, which numbers of particles are the same between two isotopes, and which ones are different? ...
... 7. With respect to the three fundamental particles of atoms, which numbers of particles are the same between two isotopes, and which ones are different? ...
PP 04 Atoms_ molecules_ ions
... Metals: Left three quarters of the chart. Lose electrons. Become positive. ...
... Metals: Left three quarters of the chart. Lose electrons. Become positive. ...
Chem vocab quiz definitons
... Valence electrons are the electrons in the outer shell and control the elements reactivity. Molecule is the smallest particle of a compound that has all the properties of the compound. Compound is a pure substance that is made of two or more elements chemically bound together. Pure substances are bo ...
... Valence electrons are the electrons in the outer shell and control the elements reactivity. Molecule is the smallest particle of a compound that has all the properties of the compound. Compound is a pure substance that is made of two or more elements chemically bound together. Pure substances are bo ...
Element Blocks Project
... Your assignment is to produce an element block that will have six sides, each having different information about your element. Elements will be assigned randomly. Your teacher will show you how to make the block after you have researched and obtained all the information that will go onto you block. ...
... Your assignment is to produce an element block that will have six sides, each having different information about your element. Elements will be assigned randomly. Your teacher will show you how to make the block after you have researched and obtained all the information that will go onto you block. ...
BellWork 2/16/2015
... In an isotope, the number of protons and electrons never changes- only the number of neutrons is different This means that each isotope of a particular element has a different atomic mass than another isotope of the same element ◦ Remember: C-12 has an atomic mass of 12 and C14 has an atomic mass of ...
... In an isotope, the number of protons and electrons never changes- only the number of neutrons is different This means that each isotope of a particular element has a different atomic mass than another isotope of the same element ◦ Remember: C-12 has an atomic mass of 12 and C14 has an atomic mass of ...
Study Guide - Honors Chemistry
... one nucleus is broken into multiple (2 in this case) nuclei by force (an alpha particle is used to break it up) one nucleus is broken into multiple (2 in this case) nuclei on its own. No force is needed. one nucleus is transformed into another nucleus by bombarding a particle into it. A particle may ...
... one nucleus is broken into multiple (2 in this case) nuclei by force (an alpha particle is used to break it up) one nucleus is broken into multiple (2 in this case) nuclei on its own. No force is needed. one nucleus is transformed into another nucleus by bombarding a particle into it. A particle may ...
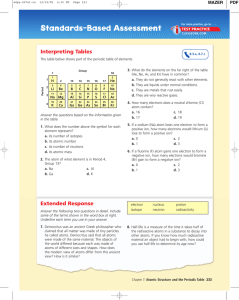
Periodic Table Vocabulary Periodic Table – a chart that organizes
... Element – a substance that cannot be broken down into a simpler substance by ordinary chemical means. Elemental Symbol – the single capital letter or capital letter followed by a lower case letter or letters that represents the name of the element. Atom – the smallest unit of an element that has all ...
... Element – a substance that cannot be broken down into a simpler substance by ordinary chemical means. Elemental Symbol – the single capital letter or capital letter followed by a lower case letter or letters that represents the name of the element. Atom – the smallest unit of an element that has all ...
Exemplar exam question – Chapter 2
... The first answer is probably worthy of only 1 mark as it does not make clear that isotopes are different atoms of the same element. The second answer would probably score 0. Although the idea of the same element and different number of neutrons is mentioned, the student has not mentioned different a ...
... The first answer is probably worthy of only 1 mark as it does not make clear that isotopes are different atoms of the same element. The second answer would probably score 0. Although the idea of the same element and different number of neutrons is mentioned, the student has not mentioned different a ...
Minerals * Chemistry Review
... • The number of protons plus neutrons gives the atom its atomic mass • All atoms of a given element have the same number of protons ...
... • The number of protons plus neutrons gives the atom its atomic mass • All atoms of a given element have the same number of protons ...
Periodic Table Review Key
... H 3. Which non-metal has the smallest atomic mass? F 4. Which metal has the largest atomic mass? D 5. Which elements are considered good conductors? C,E,D, 6. Which element is considered semi-conductors? G 7. Which elements are considered poor conductors? F, B, A,H 8. Which element is considered met ...
... H 3. Which non-metal has the smallest atomic mass? F 4. Which metal has the largest atomic mass? D 5. Which elements are considered good conductors? C,E,D, 6. Which element is considered semi-conductors? G 7. Which elements are considered poor conductors? F, B, A,H 8. Which element is considered met ...
What is the history of chemistry and elements
... 2. What is the structure of an atom? 3. How are ions formed from atoms? History 2400 year ago Greek philosophers proposed that everything was made of four basic substances – air, water, fire, and earth. Today chemists know that there are 100+ basic substances, or elements. Everything on Earth ...
... 2. What is the structure of an atom? 3. How are ions formed from atoms? History 2400 year ago Greek philosophers proposed that everything was made of four basic substances – air, water, fire, and earth. Today chemists know that there are 100+ basic substances, or elements. Everything on Earth ...
Atomic Structure - hrsbstaff.ednet.ns.ca
... So, what’s up with all these isotopes anyway? In nature elements are not made up of atoms that are all exactly the same! Some will be heavier than others, even though they are still the same type of atom. C-12 and C-14 are both Carbon, with all the usual Carbon properties, but the C-14 has two more ...
... So, what’s up with all these isotopes anyway? In nature elements are not made up of atoms that are all exactly the same! Some will be heavier than others, even though they are still the same type of atom. C-12 and C-14 are both Carbon, with all the usual Carbon properties, but the C-14 has two more ...