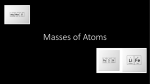* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download BellWork 2/16/2015
Survey
Document related concepts
Transcript
Mg2+ ClFe2+ O2These elements are written to describe what? Grab a textbook and look it up if needed. Isotopes are atoms that have a different number of neutrons. An atom is still the same element if it has different neutrons - it’s just a different version! Neutrons can be added to an atom without altering the number of protons and electrons it has. For example, the element carbon has 13 different isotopes! You are familiar with C-12 (the C stands for carbon, and the 12 is it’s atomic mass) ◦ This isotope has 6 neutrons C-14 (atomic mass 14) is used for carbon dating and has 8 neutrons! In an isotope, the number of protons and electrons never changes- only the number of neutrons is different This means that each isotope of a particular element has a different atomic mass than another isotope of the same element ◦ Remember: C-12 has an atomic mass of 12 and C14 has an atomic mass of 14! There are several different ways to write symbols for isotopes, each of which is perfectly legitimate. The other ways to write each of these hydrogen isotopes from top to bottom are: Hydrogen-1 or H-1 Hydrogen-2 or H-2 Hydrogen-3 or H-3 Example Write down the three different notations for a carbon atom with 6 protons and 6 neutrons. 1) 2) 3) 4) 5) Calculate the number of neutrons there are in the following isotopes (use your periodic table to find the atomic numbers) Carbon-14 Nitrogen-15 Sulfur-35 Calcium-45 Iodine-131