chapter 4 types of chemical reactions and solution stoichiometry
... a. The species reduced is the element that gains electrons. The reducing agent causes reducduction to occur by itself being oxidized. The reducing agent generally refers to the entire formula of the compound/ion that contains the element oxidized. b. The species oxidized is the element that loses el ...
... a. The species reduced is the element that gains electrons. The reducing agent causes reducduction to occur by itself being oxidized. The reducing agent generally refers to the entire formula of the compound/ion that contains the element oxidized. b. The species oxidized is the element that loses el ...
c00kieee - Ritter Illustration
... f-block elements, actinides (5f ) and lanthanides (4f ) are separated from the other elements. This modern placement as well as their name is attributed to Prof. Glenn T. Seaborg, who in the 1930s proposed the actinide theory. As a result of this concept, the actinides were removed from their origin ...
... f-block elements, actinides (5f ) and lanthanides (4f ) are separated from the other elements. This modern placement as well as their name is attributed to Prof. Glenn T. Seaborg, who in the 1930s proposed the actinide theory. As a result of this concept, the actinides were removed from their origin ...
Chromatographic Enrichment of Lithium Isotopes by Hydrous
... The chemistry of the hydrous metal oxides has been studied in a large number of scientific fields, especially, ion exchange separation, scavenging by sorption and coprecipitation, separation of mineral, nuclear fuels, antiperspirants, radioactive contamination, and scavenging of trace metals.1 The h ...
... The chemistry of the hydrous metal oxides has been studied in a large number of scientific fields, especially, ion exchange separation, scavenging by sorption and coprecipitation, separation of mineral, nuclear fuels, antiperspirants, radioactive contamination, and scavenging of trace metals.1 The h ...
Elements Compounds
... A mixture contains two or more different substances that are only physically joined together, not chemically. A mixture can contain both elements and compounds. ...
... A mixture contains two or more different substances that are only physically joined together, not chemically. A mixture can contain both elements and compounds. ...
Help us improve Wikipedia by supporting it financially
... But for international trade, the official names of the chemical elements both ancient and recent are decided by the International Union of Pure and Applied Chemistry, which has decided on a sort of international English language. That organization has recently prescribed that "aluminium" and "caesiu ...
... But for international trade, the official names of the chemical elements both ancient and recent are decided by the International Union of Pure and Applied Chemistry, which has decided on a sort of international English language. That organization has recently prescribed that "aluminium" and "caesiu ...
Masses of Atoms
... Atomic Mass Unit ~ 1/12th of the mass of one carbon-12 atom The periodic table shows the atomic mass of Nickel as 58.693. How can there be a decimal point, if the mass is whole numbers of protons and neutrons? ...
... Atomic Mass Unit ~ 1/12th of the mass of one carbon-12 atom The periodic table shows the atomic mass of Nickel as 58.693. How can there be a decimal point, if the mass is whole numbers of protons and neutrons? ...
Reading 2.1 A Return to Isotopes
... mass number, however, is A = 6 for the isotope with 3 neutrons, and A = 7 for the isotope with 4 neutrons. In nature, only certain isotopes exist. For instance, stable lithium exists as an isotope with 3 neutrons and as an isotope with 4 neutrons, but there are no stable lithium isotopes with 2 neut ...
... mass number, however, is A = 6 for the isotope with 3 neutrons, and A = 7 for the isotope with 4 neutrons. In nature, only certain isotopes exist. For instance, stable lithium exists as an isotope with 3 neutrons and as an isotope with 4 neutrons, but there are no stable lithium isotopes with 2 neut ...
Masses of Atoms
... Atomic Mass ~ number of neutrons AND number of protons Isotope ~ atoms of the same element, with different numbers of neutrons Carbon - 12 (6 protons, 6 neutrons) Carbon - 14 (6 protons, 8 neutrons) ...
... Atomic Mass ~ number of neutrons AND number of protons Isotope ~ atoms of the same element, with different numbers of neutrons Carbon - 12 (6 protons, 6 neutrons) Carbon - 14 (6 protons, 8 neutrons) ...
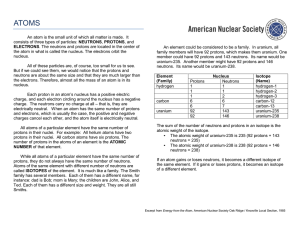
An atom is the small unit of which all matter is made. It consists of
... ELECTRONS. The neutrons and protons are located in the center of the atom in what is called the nucleus. The electrons orbit the nucleus. All of these particles are, of course, too small for us to see. But if we could see them, we would notice that the protons and neutrons are about the same size an ...
... ELECTRONS. The neutrons and protons are located in the center of the atom in what is called the nucleus. The electrons orbit the nucleus. All of these particles are, of course, too small for us to see. But if we could see them, we would notice that the protons and neutrons are about the same size an ...
Chemical Element
... Bang nucleosynthesis during the first 20 minutes of the universe[9] in a ratio of around 3:1 by mass (approximately 12:1 by number of atoms). Almost all other elements found in nature, including some further hydrogen and helium created since then, were made by various natural or (at times) artificia ...
... Bang nucleosynthesis during the first 20 minutes of the universe[9] in a ratio of around 3:1 by mass (approximately 12:1 by number of atoms). Almost all other elements found in nature, including some further hydrogen and helium created since then, were made by various natural or (at times) artificia ...
mystery elements
... What are the forces called in the nucleus that hold the protons and neutrons together, even though like charges should repel? ______________________________ Define atomic number. Define isotope (p. 76) Isotopes have a different number of which subatomic particle? __________________ Because isotopes ...
... What are the forces called in the nucleus that hold the protons and neutrons together, even though like charges should repel? ______________________________ Define atomic number. Define isotope (p. 76) Isotopes have a different number of which subatomic particle? __________________ Because isotopes ...
The atom: Isotopes (Grade 10) [NCS]
... elements as X-A where the X is the element symbol and the A is the atomic mass of that element. ...
... elements as X-A where the X is the element symbol and the A is the atomic mass of that element. ...
Atomic Number
... • Atoms are the building blocks of all materials • An atom is made of 3 parts: – Protons and Neutrons are in the nucleus (center) – Electrons orbit around the nucleus ...
... • Atoms are the building blocks of all materials • An atom is made of 3 parts: – Protons and Neutrons are in the nucleus (center) – Electrons orbit around the nucleus ...
I. The Atomic Concept:
... a. Natural radioactivity: ______________________________________________________________ ...
... a. Natural radioactivity: ______________________________________________________________ ...
Getting to Know: Periodic Table
... element do not have the same number of neutrons. For example, most atoms of the element carbon, C, have 6 neutrons in their nucleus. There are some atoms of carbon that have 7 or even 8 neutrons in their nucleus. Atoms of the same element that have a different number of neutrons are called isotopes. ...
... element do not have the same number of neutrons. For example, most atoms of the element carbon, C, have 6 neutrons in their nucleus. There are some atoms of carbon that have 7 or even 8 neutrons in their nucleus. Atoms of the same element that have a different number of neutrons are called isotopes. ...
LAB ACTIVITY CALCULATING ATOMIC MASS
... Press Release from the Virginia Beach News: Virginia Beach, VA- Chemists, working on a project for the city of Virginia Beach, have recently discovered what is believed to be element 114. The researchers have proposed that the element be named vabeachium (va-beach-e-m), after the city in which it wa ...
... Press Release from the Virginia Beach News: Virginia Beach, VA- Chemists, working on a project for the city of Virginia Beach, have recently discovered what is believed to be element 114. The researchers have proposed that the element be named vabeachium (va-beach-e-m), after the city in which it wa ...
Bean Bag Lab
... Bean BAG Isotopes Lab (5opts) Introduction: John Dalton’s atomic theory that stated all atoms of the same element are identical and equal in mass was simple yet revolutionary. Unfortunately, it was not quite right. More research started to show that atoms of the same element could have different mas ...
... Bean BAG Isotopes Lab (5opts) Introduction: John Dalton’s atomic theory that stated all atoms of the same element are identical and equal in mass was simple yet revolutionary. Unfortunately, it was not quite right. More research started to show that atoms of the same element could have different mas ...
Reading Comprehension - Easy Peasy All-in
... The list of elements is arranged on a scientific chart. The chart is called the periodic table. Each element is grouped with other similar elements. Elements can be metals, nonmetals, or semimetals. Semimetals have some of the characteristics of metals, and some of the characteristics of nonmetals. M ...
... The list of elements is arranged on a scientific chart. The chart is called the periodic table. Each element is grouped with other similar elements. Elements can be metals, nonmetals, or semimetals. Semimetals have some of the characteristics of metals, and some of the characteristics of nonmetals. M ...
Trends in the Periodic Table
... Name: _________________________________ Period: ____ Date: ___ / ___ / ___ ...
... Name: _________________________________ Period: ____ Date: ___ / ___ / ___ ...
ISOTOPES
... Why are relative atomic masses decimals, and not simple whole numbers? Dalton’s original model of an atom assumed that all atoms of each element were the same. According to the model of atomic structure we have been developing, this would mean that each atom of an element would have the same number ...
... Why are relative atomic masses decimals, and not simple whole numbers? Dalton’s original model of an atom assumed that all atoms of each element were the same. According to the model of atomic structure we have been developing, this would mean that each atom of an element would have the same number ...
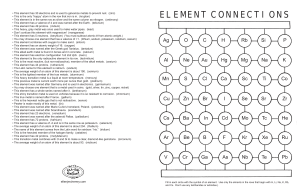
element connections
... • This element has 5 neutrons. (beryllium) (You must subtract atomic # from atomic weight.) • You may choose one element that has a valence of +1. (lithium, sodium, potassium, rubidium, cesium) • This element combines with oxygen to make sand. (silicon) • This element has an atomic weight of 16. (ox ...
... • This element has 5 neutrons. (beryllium) (You must subtract atomic # from atomic weight.) • You may choose one element that has a valence of +1. (lithium, sodium, potassium, rubidium, cesium) • This element combines with oxygen to make sand. (silicon) • This element has an atomic weight of 16. (ox ...
PS 2.2 - S2TEM Centers SC
... Easter Egg Isotopes Introduction to the lesson: Isotopes have the same atomic number and hence nearly identical chemical behavior but different atomic masses. Most elements found in nature are mixtures of several isotopes; tin, for example, has 10 isotopes. In most cases, only stable isotopes of ele ...
... Easter Egg Isotopes Introduction to the lesson: Isotopes have the same atomic number and hence nearly identical chemical behavior but different atomic masses. Most elements found in nature are mixtures of several isotopes; tin, for example, has 10 isotopes. In most cases, only stable isotopes of ele ...
Elements, Isotopes and Ions
... 2. 1 Calcium atom has 22 neutrons and another Calcium atom has 24 neutrons. Why are these both Calcium atoms even though they have a different # of neutrons? ...
... 2. 1 Calcium atom has 22 neutrons and another Calcium atom has 24 neutrons. Why are these both Calcium atoms even though they have a different # of neutrons? ...
Isotopes
... • Isotopes are atoms that have the same number of protons, but different numbers of neutrons. • They can be a radioactive form of an element. – Atoms of the same element all have the same number of protons. – Isotopes of the element have different numbers of neutrons. ...
... • Isotopes are atoms that have the same number of protons, but different numbers of neutrons. • They can be a radioactive form of an element. – Atoms of the same element all have the same number of protons. – Isotopes of the element have different numbers of neutrons. ...
isotopes
... superscript to the left of the symbol 3.) the atomic number is written as a subscript to the left. Study the illustration below ...
... superscript to the left of the symbol 3.) the atomic number is written as a subscript to the left. Study the illustration below ...
Nobelium

Nobelium is a synthetic chemical element with symbol No and atomic number 102. It is named in honor of Alfred Nobel, the inventor of dynamite and benefactor of science. A radioactive metal, it is the tenth transuranic element and is the penultimate member of the actinide series. Like all elements with atomic number over 100, nobelium can only be produced in particle accelerators by bombarding lighter elements with charged particles. A total of twelve nobelium isotopes are known to exist; the most stable is 259No with a half-life of 58 minutes, but the shorter-lived 255No (half-life 3.1 minutes) is most commonly used in chemistry because it can be produced on a larger scale.Chemistry experiments have confirmed that nobelium behaves as a heavier homolog to ytterbium in the periodic table. The chemical properties of nobelium are not completely known: they are mostly only known in aqueous solution. Before nobelium's discovery, it was predicted that it would show a stable +2 oxidation state as well as the +3 state characteristic of the other actinides: these predictions were later confirmed, as the +2 state is much more stable than the +3 state in aqueous solution and it is difficult to keep nobelium in the +3 state.In the 1950s and 1960s, many claims of the discovery of nobelium were made from laboratories in Sweden, the Soviet Union, and the United States. Although the Swedish scientists soon retracted their claims, the priority of the discovery and therefore the naming of the element was disputed between Soviet and American scientists, and it was not until 1997 that International Union of Pure and Applied Chemistry (IUPAC) established nobelium as the official name for the element and credited the Soviet team with the discovery.








![The atom: Isotopes (Grade 10) [NCS]](http://s1.studyres.com/store/data/016109524_1-1437871a54cd24e5ee13c27e98f0719d-300x300.png)