Elements and the Periodic Table
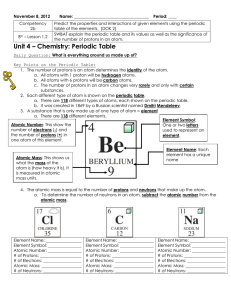
... Goal- Determine the number of protons (atomic number), number of neutrons, atomic mass, and identity of the nucleus of 5 different atoms for 5 different elements. Background (Periodic Table on page 84 and 85 in your textbook): Orange BB’s = protons and Green BB’s = neutrons • Number of protons + num ...
... Goal- Determine the number of protons (atomic number), number of neutrons, atomic mass, and identity of the nucleus of 5 different atoms for 5 different elements. Background (Periodic Table on page 84 and 85 in your textbook): Orange BB’s = protons and Green BB’s = neutrons • Number of protons + num ...
Unit 2: Atomic Concepts and Periodic Table (Level 1)
... Dmitri Mendeleev (Russia) Between 1868 and 1870, in the process of writing his book, The Principles of Chemistry, Mendeleev created a table or chart that listed the known elements according to increasing order of atomic weights. When he organized the table into horizontal rows, a pattern became appa ...
... Dmitri Mendeleev (Russia) Between 1868 and 1870, in the process of writing his book, The Principles of Chemistry, Mendeleev created a table or chart that listed the known elements according to increasing order of atomic weights. When he organized the table into horizontal rows, a pattern became appa ...
Chapter 17 Resource: Properties of Atoms and the Periodic Table
... 2. Using the microtip pipette, place 15 drops of the aluminum nitrate solution in each of the wells A1–G1. Rinse the pipette with distilled water. 3. Place 15 drops of copper nitrate solution in each of wells A2–G2 using the pipette. Rinse the pipette with distilled water. 4. Repeat step 1 for each ...
... 2. Using the microtip pipette, place 15 drops of the aluminum nitrate solution in each of the wells A1–G1. Rinse the pipette with distilled water. 3. Place 15 drops of copper nitrate solution in each of wells A2–G2 using the pipette. Rinse the pipette with distilled water. 4. Repeat step 1 for each ...
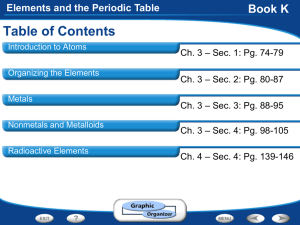
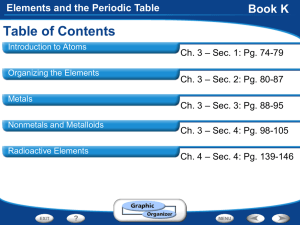
Elements and the Periodic Table
... • Elements with atomic numbers higher than 92 are sometimes described as synthetic elements because they are not found naturally on Earth. • Instead, elements that follow uranium are made – or synthesized – when nuclear particles are forced to crash into one another. • To make even heavier elements ...
... • Elements with atomic numbers higher than 92 are sometimes described as synthetic elements because they are not found naturally on Earth. • Instead, elements that follow uranium are made – or synthesized – when nuclear particles are forced to crash into one another. • To make even heavier elements ...
Chapter 2 "Elements, Atoms, and the Periodic Table"
... the water dropped, causing fish to die in large numbers. This process, called eutrophication, is considered a negative environmental impact. Today, many detergents are made without phosphorus so the detrimental effects of eutrophication are minimized. You may even see statements to that effect on de ...
... the water dropped, causing fish to die in large numbers. This process, called eutrophication, is considered a negative environmental impact. Today, many detergents are made without phosphorus so the detrimental effects of eutrophication are minimized. You may even see statements to that effect on de ...
Nucleon number
... (a) Proton number = 8, so there are 8 protons. Nucleon number = 17, so the number of neutrons is 17-8 = 9. The number of electrons is the same as the number of protons, that is 8. (b) Proton numbers = 80, so there are 80 protons. Nucleon number = 199, so the number of neutrons is 199-80 = 119. The n ...
... (a) Proton number = 8, so there are 8 protons. Nucleon number = 17, so the number of neutrons is 17-8 = 9. The number of electrons is the same as the number of protons, that is 8. (b) Proton numbers = 80, so there are 80 protons. Nucleon number = 199, so the number of neutrons is 199-80 = 119. The n ...
2. Chapter 2
... You may recall that an element is a pure substance that cannot be broken down or separated into simpler substances. The reason an element cannot be broken down further is that it is already very simple: each element is made of only one kind of atom. Elements can be found in your pencils, your coins, ...
... You may recall that an element is a pure substance that cannot be broken down or separated into simpler substances. The reason an element cannot be broken down further is that it is already very simple: each element is made of only one kind of atom. Elements can be found in your pencils, your coins, ...
Elements and the Periodic Table
... • The elements below the lanthanides are called actinides. Many of these elements are so unstable that they last for only a fraction of a second after they are made. ...
... • The elements below the lanthanides are called actinides. Many of these elements are so unstable that they last for only a fraction of a second after they are made. ...
Help us improve Wikipedia by supporting it financially
... The lightest elements are hydrogen and helium, both theoretically created by Big Bang nucleosynthesis during the first 20 minutes of the universe[10] in a ratio of around 3:1 by mass (approximately 12:1 by number of atoms). Almost all other elements found in nature, including some further hydrogen a ...
... The lightest elements are hydrogen and helium, both theoretically created by Big Bang nucleosynthesis during the first 20 minutes of the universe[10] in a ratio of around 3:1 by mass (approximately 12:1 by number of atoms). Almost all other elements found in nature, including some further hydrogen a ...
Period:______ Table Number
... 47. Nearly 2000 years ago the Greek philosopher DEMOCRITUS gave us the word atom when he said that all matter was composed of tiny indivisible particles called “atomos.” P. 73, VCR: Atoms and Molecules 48. At the present time about 118 different elements have been discovered and officially recognize ...
... 47. Nearly 2000 years ago the Greek philosopher DEMOCRITUS gave us the word atom when he said that all matter was composed of tiny indivisible particles called “atomos.” P. 73, VCR: Atoms and Molecules 48. At the present time about 118 different elements have been discovered and officially recognize ...
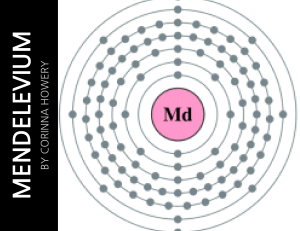
Mendelevium
... table so its atomic number is 101. There are 101 protons/electrons in the nucleus and 157 neutrons. It also has 2 valence electrons. Mendelevium has 7 shells. On the periodic table, mendelevium is in the group actinide and it is radioactive. Mendeleviums state of matter is radioactive. ...
... table so its atomic number is 101. There are 101 protons/electrons in the nucleus and 157 neutrons. It also has 2 valence electrons. Mendelevium has 7 shells. On the periodic table, mendelevium is in the group actinide and it is radioactive. Mendeleviums state of matter is radioactive. ...
Chapter 17 Resource: Properties of Atoms and the Periodic Table
... Directions: Use the terms below to complete the following paragraphs about atoms , atomic mass, and isotopes. Terms may be used more than once. six number electrons isotopes electron cloud neutron(s) proton(s) mass quarks six protons The electron has very little mass compared to the 1. _____________ ...
... Directions: Use the terms below to complete the following paragraphs about atoms , atomic mass, and isotopes. Terms may be used more than once. six number electrons isotopes electron cloud neutron(s) proton(s) mass quarks six protons The electron has very little mass compared to the 1. _____________ ...
Student Copy Study Guide Introduction to Periodic
... 19.The atomic number of an element is the total number of which particles in the nucleus? a. neutrons b. protons c. electrons d. protons and electrons 20. Who was the man who lived from 460 B.C.–370 B.C. and was among the first to suggest the idea of atoms? a. Atomos b. Dalton c. Democritus d. Thoms ...
... 19.The atomic number of an element is the total number of which particles in the nucleus? a. neutrons b. protons c. electrons d. protons and electrons 20. Who was the man who lived from 460 B.C.–370 B.C. and was among the first to suggest the idea of atoms? a. Atomos b. Dalton c. Democritus d. Thoms ...
Materials Required
... as the students take their seats. As class begins a bag of candy for the activity will be pulled out and the students will be asked what the bag contains while emphasizing what is written on the board. For today this candy will be subatomic particles. Stimulating the recall of prerequisite learning: ...
... as the students take their seats. As class begins a bag of candy for the activity will be pulled out and the students will be asked what the bag contains while emphasizing what is written on the board. For today this candy will be subatomic particles. Stimulating the recall of prerequisite learning: ...
Isotopes
... must equal the number of protons. Therefore, a sodium atom has 11 electrons in the space around its nucleus. It is always true that a sodium atom has 11 protons and 11 electrons. However, each sodium atom also has neutrons in its nucleus, and different types of sodium atoms exist that have different ...
... must equal the number of protons. Therefore, a sodium atom has 11 electrons in the space around its nucleus. It is always true that a sodium atom has 11 protons and 11 electrons. However, each sodium atom also has neutrons in its nucleus, and different types of sodium atoms exist that have different ...
1. Atomic Structure
... For some time, people thought that atoms were the smallest particles and could not be broken into anything smaller. Scientists now know that atoms are actually made from even smaller particles. There are three types: ...
... For some time, people thought that atoms were the smallest particles and could not be broken into anything smaller. Scientists now know that atoms are actually made from even smaller particles. There are three types: ...
Intro to the Periodic Table
... 7. Find the element with the atomic number of 8. a. What is the element name? _________________________ b. What is the element symbol? _________________________ c. How many protons are in one atom of this element? __________________ d. How many electrons are in one atom of this element? ____________ ...
... 7. Find the element with the atomic number of 8. a. What is the element name? _________________________ b. What is the element symbol? _________________________ c. How many protons are in one atom of this element? __________________ d. How many electrons are in one atom of this element? ____________ ...
Powerpoint covering atomic structure and isotopes
... For some time, people thought that atoms were the smallest particles and could not be broken into anything smaller. Scientists now know that atoms are actually made from even smaller particles. There are three types: ...
... For some time, people thought that atoms were the smallest particles and could not be broken into anything smaller. Scientists now know that atoms are actually made from even smaller particles. There are three types: ...
Masses of Atoms
... Atomic Number ~ number of protons in the atom of an element Atomic Mass ~ number of neutrons AND number of protons Isotope ~ atoms of the same element, with different numbers of neutrons Carbon - 12 (6 protons, 6 neutrons) Carbon - 14 (6 protons, 8 neutrons) ...
... Atomic Number ~ number of protons in the atom of an element Atomic Mass ~ number of neutrons AND number of protons Isotope ~ atoms of the same element, with different numbers of neutrons Carbon - 12 (6 protons, 6 neutrons) Carbon - 14 (6 protons, 8 neutrons) ...
OCR A Level Physics B Delivery Guide Learner Resource 1: Atomic
... © OCR 2015 - This resource may be freely copied and distributed, as long as the OCR logo and this message remain intact and OCR is acknowledged as the originator of this work. OCR acknowledges the use of the following content: Please get in touch if you want to discuss the accessibility of resources ...
... © OCR 2015 - This resource may be freely copied and distributed, as long as the OCR logo and this message remain intact and OCR is acknowledged as the originator of this work. OCR acknowledges the use of the following content: Please get in touch if you want to discuss the accessibility of resources ...
TOPIC 24 Nucleus - jmr physics website
... 16 A nucleus is represented by the symbol ~~ X. What does the nucleus contain? ...
... 16 A nucleus is represented by the symbol ~~ X. What does the nucleus contain? ...
1 Which of the following has the least mass
... 5 Which of the following statements is most accurate regarding atoms? A Most atoms cannot combine with other atoms. B Chemical reactions divide atoms into smaller units. C Atoms of the same element may have different mass numbers. D Atoms only contain protons. 6 Which part of an atom has the least m ...
... 5 Which of the following statements is most accurate regarding atoms? A Most atoms cannot combine with other atoms. B Chemical reactions divide atoms into smaller units. C Atoms of the same element may have different mass numbers. D Atoms only contain protons. 6 Which part of an atom has the least m ...
Periodic Table Extra Practice ANSWER KEY 2014
... 1.4 I can describe the charge and location of protons, neutrons, and electrons within the nucleus and shells of an atom. The periodic table is, in many ways, the world’s greatest cheat sheet. The periodic table lists all of the elements (simple substances that make up more complex materials) like go ...
... 1.4 I can describe the charge and location of protons, neutrons, and electrons within the nucleus and shells of an atom. The periodic table is, in many ways, the world’s greatest cheat sheet. The periodic table lists all of the elements (simple substances that make up more complex materials) like go ...
Flavors of the Atom
... The called those hand full of cornerstone substances elements. Between the early 1700’s and mid 1800’s chemists sought out and found over 50 of those those essential substances. At we found more and more elements we needed to ...
... The called those hand full of cornerstone substances elements. Between the early 1700’s and mid 1800’s chemists sought out and found over 50 of those those essential substances. At we found more and more elements we needed to ...
Periodic Law
... 1) and uranium (U, 92), only technetium (Tc, 43) and promethium (Pm, 61) are prepared artificially. All transuranium elements—those following uranium in the periodic table—are synthetic. Elements are produced artificially in a variety of nuclear transmutation reactions by neutrons or charged particl ...
... 1) and uranium (U, 92), only technetium (Tc, 43) and promethium (Pm, 61) are prepared artificially. All transuranium elements—those following uranium in the periodic table—are synthetic. Elements are produced artificially in a variety of nuclear transmutation reactions by neutrons or charged particl ...