Make a large atom with p:95, n:146, e:95 - TSDCurriculum
... 5. READ: This simulation only lets you to build atoms that exist in nature or have been made by scientists. If you can’t build it, it can't be made in the real world. Scientists use the word isotope to distinguish between atoms that have the same number of protons but different numbers of neutrons. ...
... 5. READ: This simulation only lets you to build atoms that exist in nature or have been made by scientists. If you can’t build it, it can't be made in the real world. Scientists use the word isotope to distinguish between atoms that have the same number of protons but different numbers of neutrons. ...
EXPERIMENT 4 – The Periodic Table
... In this experiment, you will be looking at some elements in the laboratory display. Some look different from each other, while others look similar. Elements can be categorized in several ways. In this experiment, you are going to group elements by similarities in their physical properties. Elements ...
... In this experiment, you will be looking at some elements in the laboratory display. Some look different from each other, while others look similar. Elements can be categorized in several ways. In this experiment, you are going to group elements by similarities in their physical properties. Elements ...
EXPERIMENT 4 – The Periodic Table
... In this experiment, you will be looking at some elements in the laboratory display. Some look different from each other, while others look similar. Elements can be categorized in several ways. In this experiment, you are going to group elements by similarities in their physical properties. Elements ...
... In this experiment, you will be looking at some elements in the laboratory display. Some look different from each other, while others look similar. Elements can be categorized in several ways. In this experiment, you are going to group elements by similarities in their physical properties. Elements ...
Introduction to Atoms
... • Atoms are the building blocks of all materials • An atom is made of 3 parts: – Protons and Neutrons are in the nucleus (center) – Electrons orbit around the nucleus ...
... • Atoms are the building blocks of all materials • An atom is made of 3 parts: – Protons and Neutrons are in the nucleus (center) – Electrons orbit around the nucleus ...
File
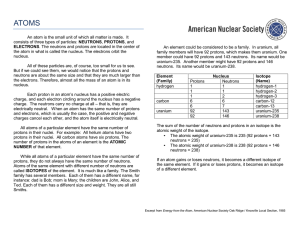
... All atoms of the same element will always have the same number of protons. Protons determine the identity of the element. Different atoms of an element may have different numbers of electrons; this forms ions. Atoms may also differ in their number of neutrons, creating isotopes. Isotopes of the same ...
... All atoms of the same element will always have the same number of protons. Protons determine the identity of the element. Different atoms of an element may have different numbers of electrons; this forms ions. Atoms may also differ in their number of neutrons, creating isotopes. Isotopes of the same ...
Nucleus Protons Neutrons Electron Cloud Electrons
... Protons and Neutrons are roughly equal in mass but a neutron is ever so _____________________ bigger than a proton. ...
... Protons and Neutrons are roughly equal in mass but a neutron is ever so _____________________ bigger than a proton. ...
IPS Unit 8 – Periodic Table Review Worksheet
... 8. The unit of measurement used for atomic particles is the (atom size/atomic mass unit). 9. Atoms of the same element that have different numbers of neutrons are called (isotopes/electron clouds). 10. In the periodic table, elements are arranged by increasing atomic (power/number). 11. An electron ...
... 8. The unit of measurement used for atomic particles is the (atom size/atomic mass unit). 9. Atoms of the same element that have different numbers of neutrons are called (isotopes/electron clouds). 10. In the periodic table, elements are arranged by increasing atomic (power/number). 11. An electron ...
SECTION REVIEW
... ________ 11. The atomic number of an element is the sum of the protons and electrons in an atom of that element. ________ 12. The atomic number of an atom is the total number of protons in an atom of that element. ________ 13. An atom of nitrogen has 7 protons and 7 neutrons. ________ 14. Relative a ...
... ________ 11. The atomic number of an element is the sum of the protons and electrons in an atom of that element. ________ 12. The atomic number of an atom is the total number of protons in an atom of that element. ________ 13. An atom of nitrogen has 7 protons and 7 neutrons. ________ 14. Relative a ...
An atom is the small unit of which all matter is made. It consists of
... ELECTRONS. The neutrons and protons are located in the center of the atom in what is called the nucleus. The electrons orbit the nucleus. All of these particles are, of course, too small for us to see. But if we could see them, we would notice that the protons and neutrons are about the same size an ...
... ELECTRONS. The neutrons and protons are located in the center of the atom in what is called the nucleus. The electrons orbit the nucleus. All of these particles are, of course, too small for us to see. But if we could see them, we would notice that the protons and neutrons are about the same size an ...
Ch 30 Nuclear Physics
... other. Therefore the repulsive forces in a nucleus are huge and the nucleus should fly apart. There must exist a third fundamental force that somehow holds the nucleus together. That 3rd force is the strong nuclear force. It is much stronger than the electromagnetic force. But strong only at short r ...
... other. Therefore the repulsive forces in a nucleus are huge and the nucleus should fly apart. There must exist a third fundamental force that somehow holds the nucleus together. That 3rd force is the strong nuclear force. It is much stronger than the electromagnetic force. But strong only at short r ...
DO NOW - PBworks
... charges, and locations, of protons and neutrons in the nucleus and electrons in the electron cloud 8.5 (B) Identify that protons determine an element’s identity and valence electrons determine its chemical properties, including reactivity ...
... charges, and locations, of protons and neutrons in the nucleus and electrons in the electron cloud 8.5 (B) Identify that protons determine an element’s identity and valence electrons determine its chemical properties, including reactivity ...
Atom - Images
... • He proposed Coulomb Forces – attractions that exist between oppositely electrically charged particles (protons & electrons) within a single atom. • The forces directly affect the atomic radii of an atom. ...
... • He proposed Coulomb Forces – attractions that exist between oppositely electrically charged particles (protons & electrons) within a single atom. • The forces directly affect the atomic radii of an atom. ...
Discussion Notes (cont.)
... Nucleus: The dense, positively charged structure found in the center of the atom. It is composed of protons and neutrons. Proton: A particle with a positive charge, found in the nucleus of atoms. Electron: A particle with a negative charge. Electrons move very fast around the outside of the nucleus ...
... Nucleus: The dense, positively charged structure found in the center of the atom. It is composed of protons and neutrons. Proton: A particle with a positive charge, found in the nucleus of atoms. Electron: A particle with a negative charge. Electrons move very fast around the outside of the nucleus ...
LBC1_Sec3_Unit01_Alchemy
... Nucleus: The dense, positively charged structure found in the center of the atom. It is composed of protons and neutrons. Proton: A particle with a positive charge, found in the nucleus of atoms. Electron: A particle with a negative charge. Electrons move very fast around the outside of the nucleus ...
... Nucleus: The dense, positively charged structure found in the center of the atom. It is composed of protons and neutrons. Proton: A particle with a positive charge, found in the nucleus of atoms. Electron: A particle with a negative charge. Electrons move very fast around the outside of the nucleus ...
Topic 1 - Periodic Table
... nitrogen, naturally occur as diatomic molecules. Matter is classified by its chemical and physical properties. Physical properties refer to the condition or quality of a substance than can be observed or measured without changed the substance’s composition. Important physical properties are density, ...
... nitrogen, naturally occur as diatomic molecules. Matter is classified by its chemical and physical properties. Physical properties refer to the condition or quality of a substance than can be observed or measured without changed the substance’s composition. Important physical properties are density, ...
Chapter 18 Comparing Atoms Lab
... In this investigation, you will investigate the structure of the atom and identify what makes atoms of different elements different from each other. We once believed that atoms were the smallest unit of matter. Then it was discovered that there are even smaller particles inside atoms (J.J. Thomson). ...
... In this investigation, you will investigate the structure of the atom and identify what makes atoms of different elements different from each other. We once believed that atoms were the smallest unit of matter. Then it was discovered that there are even smaller particles inside atoms (J.J. Thomson). ...
isotopes
... Can we write isotopes in a different way? • You can also use the mass number and the name of the element to designate the atom or isotope – This is called hyphen notation • For example, two isotopes of carbon are carbon-12 and carbon-13 – The nuclear symbols for these two isotopes would be: ...
... Can we write isotopes in a different way? • You can also use the mass number and the name of the element to designate the atom or isotope – This is called hyphen notation • For example, two isotopes of carbon are carbon-12 and carbon-13 – The nuclear symbols for these two isotopes would be: ...
File
... A fourth part, the nucleus, is often referred to but we are not focusing on it as much as we are on protons, neutrons, and electrons. The nucleus is the central part of the atom. Protons and neutrons are located in the nucleus or central part of an atom. Electrons constantly orbit around the nucleus ...
... A fourth part, the nucleus, is often referred to but we are not focusing on it as much as we are on protons, neutrons, and electrons. The nucleus is the central part of the atom. Protons and neutrons are located in the nucleus or central part of an atom. Electrons constantly orbit around the nucleus ...
Half-Life - Chemistry 1 at NSBHS
... pressure temperature concentration number of neutrons in nucleus ANS: D ...
... pressure temperature concentration number of neutrons in nucleus ANS: D ...
Atomic Number
... • Atoms are the building blocks of all materials • An atom is made of 3 parts: – Protons and Neutrons are in the nucleus (center) – Electrons orbit around the nucleus ...
... • Atoms are the building blocks of all materials • An atom is made of 3 parts: – Protons and Neutrons are in the nucleus (center) – Electrons orbit around the nucleus ...
Any substance that cannot be decomposed into
... Understanding the Elements Any substance that cannot be decomposed into simpler substances by ordinary chemical processes is defined as a chemical element. Only 94 such substances are known to exist in nature. They are found either chemically free, such as the oxygen in air, or combined with other e ...
... Understanding the Elements Any substance that cannot be decomposed into simpler substances by ordinary chemical processes is defined as a chemical element. Only 94 such substances are known to exist in nature. They are found either chemically free, such as the oxygen in air, or combined with other e ...
Reading Comprehension - Easy Peasy All-in
... matter. Elements join together with other elements to make the different materials that we see and use every day. Some common elements that you might have heard about are oxygen, carbon, helium, gold and silver. If you have a lump of silver, all of the atoms that make up that lump of silver are the ...
... matter. Elements join together with other elements to make the different materials that we see and use every day. Some common elements that you might have heard about are oxygen, carbon, helium, gold and silver. If you have a lump of silver, all of the atoms that make up that lump of silver are the ...
Multiple Choice - EDU360ScienceMethods
... properties? The periodic table is set up into columns and rows. The columns are known as groups or families. The vertical rows are called periods. The elements that share a period have the same number of atomic orbitals. The elements that are within the same group have the same number of electrons i ...
... properties? The periodic table is set up into columns and rows. The columns are known as groups or families. The vertical rows are called periods. The elements that share a period have the same number of atomic orbitals. The elements that are within the same group have the same number of electrons i ...
ATOMIC STRUCTURE questions
... The MASS of an atom is contained in the NUCLEUS, the ATOMIC MASS or MASS NUMBER of an element is the SUM of the PROTONS and the NEUTRONS. Find the element SODIUM (Na) its ATOMIC NUMBER is 11, it has 11 PROTONS in its NUCLEUS and 11 ELECTRONS outside the nucleus. Its MASS NUMBER is 23, this means tha ...
... The MASS of an atom is contained in the NUCLEUS, the ATOMIC MASS or MASS NUMBER of an element is the SUM of the PROTONS and the NEUTRONS. Find the element SODIUM (Na) its ATOMIC NUMBER is 11, it has 11 PROTONS in its NUCLEUS and 11 ELECTRONS outside the nucleus. Its MASS NUMBER is 23, this means tha ...