Atomic Number
... -Just like the 26 letters of the alphabet combine to form all the words in the English language, the 100 or so elements combine to form everything that exists in the world -About 90 of the elements on the PT are found naturally in nature, the rest have been created in a laboratory -Any material made ...
... -Just like the 26 letters of the alphabet combine to form all the words in the English language, the 100 or so elements combine to form everything that exists in the world -About 90 of the elements on the PT are found naturally in nature, the rest have been created in a laboratory -Any material made ...
study guide for atoms/periodic table quiz
... Period ROWS in the Periodic Table are called periods. The elements in a period have very different properties. Family/Group COLUMNS in the Periodic Table represent groups or families. Elements in the same family have similar properties. Bohr Diagram A drawing which shows electrons in their energy le ...
... Period ROWS in the Periodic Table are called periods. The elements in a period have very different properties. Family/Group COLUMNS in the Periodic Table represent groups or families. Elements in the same family have similar properties. Bohr Diagram A drawing which shows electrons in their energy le ...
Chapter 10 Test A
... 1. This scientist published a detailed atomic theory in 1808 based on evidence he gathered through experiments with gases. His atomic theory laid the groundwork for later atomic models. a. James Chadwick b. John Dalton c. Ernest Rutherford d. J. J. Thomson 2. One kind of particle that makes up the a ...
... 1. This scientist published a detailed atomic theory in 1808 based on evidence he gathered through experiments with gases. His atomic theory laid the groundwork for later atomic models. a. James Chadwick b. John Dalton c. Ernest Rutherford d. J. J. Thomson 2. One kind of particle that makes up the a ...
Isotope Worksheet
... On the other; hand, if we add an extra NEUTRON to a nucleus we simply end up with the same element, just a little heavier, since the charge on the nucleus would be unchanged. ...
... On the other; hand, if we add an extra NEUTRON to a nucleus we simply end up with the same element, just a little heavier, since the charge on the nucleus would be unchanged. ...
Isotope Worksheet
... On the other; hand, if we add an extra NEUTRON to a nucleus we simply end up with the same element, just a little heavier, since the charge on the nucleus would be unchanged. ! ...
... On the other; hand, if we add an extra NEUTRON to a nucleus we simply end up with the same element, just a little heavier, since the charge on the nucleus would be unchanged. ! ...
Ch 3 studentElements Ions Isotopes
... 2. all atoms of a particular element are identical 3. different elements have different atoms 4. atoms combine in certain whole-number ratios 5. In a chemical reaction, atoms are merely rearranged to form new compounds; they are not created, destroyed, or changed into atoms of any other elements ...
... 2. all atoms of a particular element are identical 3. different elements have different atoms 4. atoms combine in certain whole-number ratios 5. In a chemical reaction, atoms are merely rearranged to form new compounds; they are not created, destroyed, or changed into atoms of any other elements ...
Big History Chemistry Study Guide File
... 5. The atomic mass is equal to the ___________________ plus _____________________. 6. Every atom of _________________ in the universe has 5 protons. The atomic mass of this element is listed as _________ amu, which means that its most common isotopes have masses of _____ and _____. These isotopes ha ...
... 5. The atomic mass is equal to the ___________________ plus _____________________. 6. Every atom of _________________ in the universe has 5 protons. The atomic mass of this element is listed as _________ amu, which means that its most common isotopes have masses of _____ and _____. These isotopes ha ...
22-Introduction to Radioactivity
... isotopes in many ways. Some are beneficial (good), such as medical treatment and creating electrical energy. Others are harmful and dangerous, such as atomic bombs. In this activity, you will first review some of the important ideas about atoms. Then you will begin to learn about two important ways ...
... isotopes in many ways. Some are beneficial (good), such as medical treatment and creating electrical energy. Others are harmful and dangerous, such as atomic bombs. In this activity, you will first review some of the important ideas about atoms. Then you will begin to learn about two important ways ...
Name: Chapter 4 and 5 Study Guide Who was the Greek
... 16. What is going on inside the atoms when a neon light glows? 17. In a periodic table, a set of properties repeats from… a. Element to element b. Group to group c. Column to column d. Row to row 18. The usefulness of Mendeleev’s periodic table was confirmed by… a. The discovery of subatomic particl ...
... 16. What is going on inside the atoms when a neon light glows? 17. In a periodic table, a set of properties repeats from… a. Element to element b. Group to group c. Column to column d. Row to row 18. The usefulness of Mendeleev’s periodic table was confirmed by… a. The discovery of subatomic particl ...
Atomic Structure
... number of each type of atom on each side, nuclear equations need the same number of each type of nucleon on each side. ...
... number of each type of atom on each side, nuclear equations need the same number of each type of nucleon on each side. ...
Atomic Structure
... •Isotope is the same element with different number of ________________________ therefore, the mass number will be different for the same element. All atoms of an element are considered an isotope, some are more common than others. •Ion is same element with a different number of __________________. A ...
... •Isotope is the same element with different number of ________________________ therefore, the mass number will be different for the same element. All atoms of an element are considered an isotope, some are more common than others. •Ion is same element with a different number of __________________. A ...
are made up of
... Severalscientists,including Newlands, Meyer, and Mendeleevworked on classificationsystems that grouped elements accordingto their properties. They found that these properties repeated in a regular or periodic manner. This fact was used to predict properties of undiscovered elements. Reviewelectron a ...
... Severalscientists,including Newlands, Meyer, and Mendeleevworked on classificationsystems that grouped elements accordingto their properties. They found that these properties repeated in a regular or periodic manner. This fact was used to predict properties of undiscovered elements. Reviewelectron a ...
Structure-Prop of Matter session
... A solution is different from other types of matter like an element, compound, or mixture. A solution is a mixture of two or more substances where all parts are identical. The parts will not settle out upon standing and cannot be filtered. Yet, they are not chemically combined like in a chemical reac ...
... A solution is different from other types of matter like an element, compound, or mixture. A solution is a mixture of two or more substances where all parts are identical. The parts will not settle out upon standing and cannot be filtered. Yet, they are not chemically combined like in a chemical reac ...
The Periodic Table
... Mass number is the count of nucleons in an isotope and atomic mass is the measure of the average mass of an atom including the relative abundance of its element’s isotopes. ...
... Mass number is the count of nucleons in an isotope and atomic mass is the measure of the average mass of an atom including the relative abundance of its element’s isotopes. ...
Test Review Answers File
... 8. Why are the Noble Gases considered “inert” gases? List all the elements in this group. -undergo fewest chemical reactions, low chemical reactivity -helium, neon, argon, krypton, xenon, radon 9. List which elements are chemically similar to Beryllium. Explain why. Magnesium, calcium, strontium, ba ...
... 8. Why are the Noble Gases considered “inert” gases? List all the elements in this group. -undergo fewest chemical reactions, low chemical reactivity -helium, neon, argon, krypton, xenon, radon 9. List which elements are chemically similar to Beryllium. Explain why. Magnesium, calcium, strontium, ba ...
What is the history of chemistry and elements
... 2. What is the structure of an atom? 3. How are ions formed from atoms? History 2400 year ago Greek philosophers proposed that everything was made of four basic substances – air, water, fire, and earth. Today chemists know that there are 100+ basic substances, or elements. Everything on Earth ...
... 2. What is the structure of an atom? 3. How are ions formed from atoms? History 2400 year ago Greek philosophers proposed that everything was made of four basic substances – air, water, fire, and earth. Today chemists know that there are 100+ basic substances, or elements. Everything on Earth ...
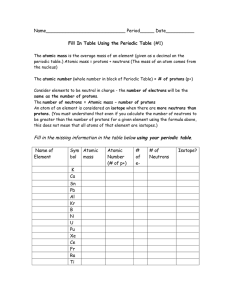
Periodic Table Fill in Table 1
... The atomic mass is the average mass of an element (given as a decimal on the periodic table.) Atomic mass = protons + neutrons (The mass of an atom comes from the nucleus) The atomic number (whole number in block of Periodic Table) = # of protons (p+) Consider elements to be neutral in charge - the ...
... The atomic mass is the average mass of an element (given as a decimal on the periodic table.) Atomic mass = protons + neutrons (The mass of an atom comes from the nucleus) The atomic number (whole number in block of Periodic Table) = # of protons (p+) Consider elements to be neutral in charge - the ...
Test 2 Review Test 2 Review (15-16)_2
... (17) ____________ Which column from above contains VERY non-reactive elements? (18) ____________ How many of these elements are gases at 0 degrees Celsius? (19) ____________ How many of these elements are metalloids? (20) ____________ How many of these elements are NON-metals and solids? (21) ______ ...
... (17) ____________ Which column from above contains VERY non-reactive elements? (18) ____________ How many of these elements are gases at 0 degrees Celsius? (19) ____________ How many of these elements are metalloids? (20) ____________ How many of these elements are NON-metals and solids? (21) ______ ...
ISOTOPES 3 SUBATOMIC PARTICLES Proton Located inside the
... Located outside of the nucleus in an “electron cloud” Involved in chemical bonding Negative charge Equal to the # of protons in a neutral atom How many electrons does Potassium have? How many electrons does Nitrogen have? o Neutron Located inside the nucleus of an atom No charge # ...
... Located outside of the nucleus in an “electron cloud” Involved in chemical bonding Negative charge Equal to the # of protons in a neutral atom How many electrons does Potassium have? How many electrons does Nitrogen have? o Neutron Located inside the nucleus of an atom No charge # ...
Atomic Structure
... Different atoms have different ____________ and ____________ The differing properties of matter are due to the size, shape, and movement of ____________ Changes in matter result from changes in the ____________ of atoms and not the atoms themselves ...
... Different atoms have different ____________ and ____________ The differing properties of matter are due to the size, shape, and movement of ____________ Changes in matter result from changes in the ____________ of atoms and not the atoms themselves ...
PS 2.2
... the weighted average of the masses of the naturally occurring isotopes of an element. The atomic mass of an element can be found on the periodic table. Since it is an average, it is usually not a whole number. ...
... the weighted average of the masses of the naturally occurring isotopes of an element. The atomic mass of an element can be found on the periodic table. Since it is an average, it is usually not a whole number. ...