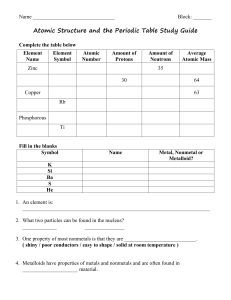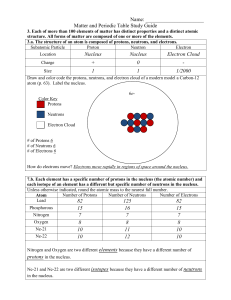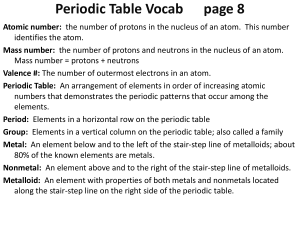
I can describe an atom and its components I can relate energy levels
... ● The second energy level can hold 8 electrons ● The third energy level can hold 18 electrons ● The fourth energy level can hold 32 electrons. ...
... ● The second energy level can hold 8 electrons ● The third energy level can hold 18 electrons ● The fourth energy level can hold 32 electrons. ...
Name: Chapter 4 and 5 Study Guide Who was the Greek
... 16. What is going on inside the atoms when a neon light glows? 17. In a periodic table, a set of properties repeats from… a. Element to element b. Group to group c. Column to column d. Row to row 18. The usefulness of Mendeleev’s periodic table was confirmed by… a. The discovery of subatomic particl ...
... 16. What is going on inside the atoms when a neon light glows? 17. In a periodic table, a set of properties repeats from… a. Element to element b. Group to group c. Column to column d. Row to row 18. The usefulness of Mendeleev’s periodic table was confirmed by… a. The discovery of subatomic particl ...
Review 1st Qtr KEY
... CONCEPT QUESTIONS: Identify the letter of the choice that best completes the statement or answers the question. ANSWERS: A, B, B, B, B, B, C, C ____ 1. Most of the mass of an atom is found a. In the electron cloud. c. in the number of protons. b. in the nucleus. d. in the outer region of an atom. __ ...
... CONCEPT QUESTIONS: Identify the letter of the choice that best completes the statement or answers the question. ANSWERS: A, B, B, B, B, B, C, C ____ 1. Most of the mass of an atom is found a. In the electron cloud. c. in the number of protons. b. in the nucleus. d. in the outer region of an atom. __ ...
Understanding the Atom GN
... When atoms of the same element have different numbers of neutrons they are called ____________________. Isotope – ________________________________________________________________________ Most elements have ______________________ isotopes. Mass Number - ________________________________________ ...
... When atoms of the same element have different numbers of neutrons they are called ____________________. Isotope – ________________________________________________________________________ Most elements have ______________________ isotopes. Mass Number - ________________________________________ ...
CH.2
... identify key groups, periods, and regions of elements on the periodic table. (B1) identify and explain trends in the periodic table as they relate to ionization energy, electronegativity, shielding effect, and relative (B1, B2) compare an element’s reactivity to the reactivity of other element ...
... identify key groups, periods, and regions of elements on the periodic table. (B1) identify and explain trends in the periodic table as they relate to ionization energy, electronegativity, shielding effect, and relative (B1, B2) compare an element’s reactivity to the reactivity of other element ...
and View
... A. 7 periods—rows of elements whose properties change gradually and predictably. B. 18 groups---columns with family of elements having similar properties both physical and chemical. C. Group 1 and 2, 13 to 18—representative elements. a. Metals b. Nonmetals c. Metalloids D. Group 3 to 12—transition e ...
... A. 7 periods—rows of elements whose properties change gradually and predictably. B. 18 groups---columns with family of elements having similar properties both physical and chemical. C. Group 1 and 2, 13 to 18—representative elements. a. Metals b. Nonmetals c. Metalloids D. Group 3 to 12—transition e ...
Ions
... particle called an ion. When an atom loses an electron it has more protons therefore becoming positively charged. When an atom gains an electron it has more electrons therefore becoming negatively charged. ...
... particle called an ion. When an atom loses an electron it has more protons therefore becoming positively charged. When an atom gains an electron it has more electrons therefore becoming negatively charged. ...
Slide 1
... We cannot show videos due to copyright. Sources for appropriate videos may include: Discovery Education and AGC Educational Media ...
... We cannot show videos due to copyright. Sources for appropriate videos may include: Discovery Education and AGC Educational Media ...
The History of the Modern Periodic Table
... In 1913, through his work with X-rays, he determined the actual nuclear charge (atomic number) of the elements*. He rearranged the elements in order of increasing atomic number. *“There is in the atom a fundamental quantity which increases by regular steps as we pass from each element to the next. T ...
... In 1913, through his work with X-rays, he determined the actual nuclear charge (atomic number) of the elements*. He rearranged the elements in order of increasing atomic number. *“There is in the atom a fundamental quantity which increases by regular steps as we pass from each element to the next. T ...
The study of biology can help you better understand human
... Which subatomic particle determines chemical properties of an element? ...
... Which subatomic particle determines chemical properties of an element? ...
Chemistry Exam Review
... Isotope • an atom with a different number of neutrons and therefore a different mass ...
... Isotope • an atom with a different number of neutrons and therefore a different mass ...
Extra Credit Test Review
... 12.One atom has 17 protons, 18 neutrons, and 17 electrons. Another atom has 17 protons, 19 neutrons and 17 electrons. Are these the same element? Yes No Explain: __________________________________________________________________ 13.Today we use Mendeleev’s arrangement, elements are arranged by incre ...
... 12.One atom has 17 protons, 18 neutrons, and 17 electrons. Another atom has 17 protons, 19 neutrons and 17 electrons. Are these the same element? Yes No Explain: __________________________________________________________________ 13.Today we use Mendeleev’s arrangement, elements are arranged by incre ...
Semester 1 Exam Review Part 1
... there are more protons than electrons-the charge of the atom will be positive ...
... there are more protons than electrons-the charge of the atom will be positive ...
CHEM 101 Dual Enrollment HW4 Question 1 of 12 Dalton`s
... Question 1 of 12 Dalton's postulates, also known as Dalton's atomic theory, include some proposals that have been updated or changed due to new discoveries. Which of the following statements were parts of Dalton's original atomic theory? Select all that apply. Atoms of the same element have the same ...
... Question 1 of 12 Dalton's postulates, also known as Dalton's atomic theory, include some proposals that have been updated or changed due to new discoveries. Which of the following statements were parts of Dalton's original atomic theory? Select all that apply. Atoms of the same element have the same ...
Chemical reactions revision
... Element are arranged in the table in order of their atomic number Elements in different groups (columns) have different properties. Elements are often split into the groups metals and non-metals. Metals are strong, sonorous (ring), malleable (can be bent into shape) and are good conductors of heat a ...
... Element are arranged in the table in order of their atomic number Elements in different groups (columns) have different properties. Elements are often split into the groups metals and non-metals. Metals are strong, sonorous (ring), malleable (can be bent into shape) and are good conductors of heat a ...
Exam 1 Review Questions
... Covalent compounds contain both metal and nonmetal atoms. Ionic compounds are made of molecules. Dmitri Mendeleev published the first modern atomic theory in 1805. Fluorine is found as a metal in its pure form. Francium chloride FrCl is a covalent compound. Graphite is a compound containing carbon a ...
... Covalent compounds contain both metal and nonmetal atoms. Ionic compounds are made of molecules. Dmitri Mendeleev published the first modern atomic theory in 1805. Fluorine is found as a metal in its pure form. Francium chloride FrCl is a covalent compound. Graphite is a compound containing carbon a ...
Notes
... -the number of protons in an atom of an element •all atoms of an element have the same atomic # •written as a subscript next to the element’s symbol •in a neutral atom, the number of protons is equal to the number of electrons (balanced charges). ...
... -the number of protons in an atom of an element •all atoms of an element have the same atomic # •written as a subscript next to the element’s symbol •in a neutral atom, the number of protons is equal to the number of electrons (balanced charges). ...
Chapter 11 and 12-2 Review/Study Guide for Test
... 13. Explain the difference between a group and a period on the periodic table of elements. Groups = columns (there are 18 total) and Periods = Rows (there are 7). Also, a group shares similar properties. 14. Why are neither the alkali metals nor the alkaline-earth metals found uncombined in nature? ...
... 13. Explain the difference between a group and a period on the periodic table of elements. Groups = columns (there are 18 total) and Periods = Rows (there are 7). Also, a group shares similar properties. 14. Why are neither the alkali metals nor the alkaline-earth metals found uncombined in nature? ...
2.2 Periodic Trends
... What are the trends that occur in the periodic table by organizing elements by their atomic number? Periodic trends are specific patterns that are present in the periodic table that illustrate different aspects of a certain element. Periodic trends, arising from the arrangement of the periodic t ...
... What are the trends that occur in the periodic table by organizing elements by their atomic number? Periodic trends are specific patterns that are present in the periodic table that illustrate different aspects of a certain element. Periodic trends, arising from the arrangement of the periodic t ...