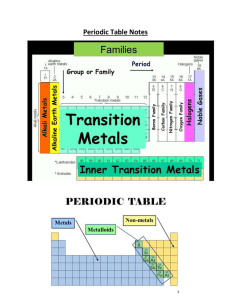
Unit 4 Study Guide Groups to know: Alkali Metals
... F (fluorine) has the highest electronegativity and is the most reactive of all nonmetals *Check your notes to know WHY these trends occur! You must know this!!!* ...
... F (fluorine) has the highest electronegativity and is the most reactive of all nonmetals *Check your notes to know WHY these trends occur! You must know this!!!* ...
Chap 7: Around the Room Review
... 1. The central part of an atom is called the _____ 2. A proton has a _____ charge. 3. The atomic number tells us __________. 4. Nitrogen’s atomic number is 7. An isotope of nitrogen containing 7 neutrons would be nitrogen_____. 5. How does the size of a negative ion compare to the size of the atom t ...
... 1. The central part of an atom is called the _____ 2. A proton has a _____ charge. 3. The atomic number tells us __________. 4. Nitrogen’s atomic number is 7. An isotope of nitrogen containing 7 neutrons would be nitrogen_____. 5. How does the size of a negative ion compare to the size of the atom t ...
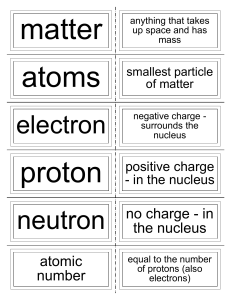
Atom
... 1904: J.J.Thompson discovered electrons & proposed the “plum pudding model” 1911: Earnest Rutherford discovered the nucleus. 1913: Neils Bohr proposed that electrons orbit with electrostic forces rather than gravity. the “planetary model” 1926: Erwin Schrodinger analyzed electron orbits from a geome ...
... 1904: J.J.Thompson discovered electrons & proposed the “plum pudding model” 1911: Earnest Rutherford discovered the nucleus. 1913: Neils Bohr proposed that electrons orbit with electrostic forces rather than gravity. the “planetary model” 1926: Erwin Schrodinger analyzed electron orbits from a geome ...
Unit 3 Notebook Notes
... o He was wrong, but his theory persisted for 2000 years 1808 John Dalton developed the “Atomic Theory” based on Experiments 1. All matter is made of small particles called atoms. 2. Atoms of an element are identical in size, mass and other properties. Atoms of different elements differ in size, ma ...
... o He was wrong, but his theory persisted for 2000 years 1808 John Dalton developed the “Atomic Theory” based on Experiments 1. All matter is made of small particles called atoms. 2. Atoms of an element are identical in size, mass and other properties. Atoms of different elements differ in size, ma ...
Ions and isotopes
... • An ISOTOPE is a form of an element that has a different number of neutrons than “normal” • Carbon has three isotopes ...
... • An ISOTOPE is a form of an element that has a different number of neutrons than “normal” • Carbon has three isotopes ...
Dmitri MendeleevанааA Russian chemist, noticed a repeating
... pattern of chemical properties in the elements that were known at the time. Mendeleev arranged the elements in the order of increasing atomic mass to form something close to the modern day periodic table. The pattern of repeating order is called periodicity. ...
... pattern of chemical properties in the elements that were known at the time. Mendeleev arranged the elements in the order of increasing atomic mass to form something close to the modern day periodic table. The pattern of repeating order is called periodicity. ...
Chapter 7 Review Sheet
... creature approaches you. He explains that he is a scientist from planet Rainhard in a far corner of the universe. Only 12 elements are known to exist on Rainhard. He has been trying unsuccessfully to organize a periodic table for these elements, and in desperation has come to the far-off planet Eart ...
... creature approaches you. He explains that he is a scientist from planet Rainhard in a far corner of the universe. Only 12 elements are known to exist on Rainhard. He has been trying unsuccessfully to organize a periodic table for these elements, and in desperation has come to the far-off planet Eart ...
Exam 3 Review - Iowa State University
... d. Atomic radius, K or Cs e. Atomic radius, Se or Br 12. List three properties that distinguish nonmetals from metals. 13. Which of the following are solids at room temperature, and which are gases? a. CO2 b. BaO c. CuO d. F2 e. NO 14. Which substances are ionic and which are covalent? a. Br2 b. KO2 ...
... d. Atomic radius, K or Cs e. Atomic radius, Se or Br 12. List three properties that distinguish nonmetals from metals. 13. Which of the following are solids at room temperature, and which are gases? a. CO2 b. BaO c. CuO d. F2 e. NO 14. Which substances are ionic and which are covalent? a. Br2 b. KO2 ...
File
... nucleus of an atom. (The number of protons in the nucleus is the atomic number, which determines the identity of an element.) neutron: a subatomic particle that has no charge and that is located in the nucleus of an atom. electron: a subatomic particle that has a negative charge. element: a substanc ...
... nucleus of an atom. (The number of protons in the nucleus is the atomic number, which determines the identity of an element.) neutron: a subatomic particle that has no charge and that is located in the nucleus of an atom. electron: a subatomic particle that has a negative charge. element: a substanc ...
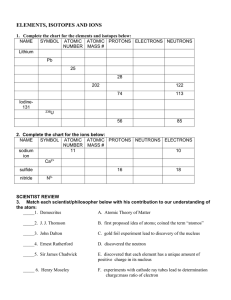
ELEMENTS, ISOTOPES AND IONS
... 1. Complete the chart for the elements and isotopes below: NAME SYMBOL ATOMIC ATOMIC PROTONS ELECTRONS NEUTRONS NUMBER MASS # ...
... 1. Complete the chart for the elements and isotopes below: NAME SYMBOL ATOMIC ATOMIC PROTONS ELECTRONS NEUTRONS NUMBER MASS # ...
Period Table, valence Electrons and Ion Notes
... Example: Na = 1s2 2s2 2p6 3s1 Add up the e-‘s found in the last energy level, in this case there is only 1 so Na has 1 valence e**You have to do this for the Transition metal every time** ...
... Example: Na = 1s2 2s2 2p6 3s1 Add up the e-‘s found in the last energy level, in this case there is only 1 so Na has 1 valence e**You have to do this for the Transition metal every time** ...
Atoms, Molecules and Ions
... Dalton’s Atomic Theory (1808) (Listed on p 203) 1. Elements are composed of extremely small particles called atoms. 2. All atoms of a given element are identical, having the same size, mass and chemical properties. 3. The atoms of one element are different from the atoms of all other elements. 4. C ...
... Dalton’s Atomic Theory (1808) (Listed on p 203) 1. Elements are composed of extremely small particles called atoms. 2. All atoms of a given element are identical, having the same size, mass and chemical properties. 3. The atoms of one element are different from the atoms of all other elements. 4. C ...
Power point on the Periodic Table
... Neutron: in the nucleus, symbol “n”, no charge, slightly larger mass than a proton ...
... Neutron: in the nucleus, symbol “n”, no charge, slightly larger mass than a proton ...
Chemistry Notes
... sodium, magnesium, aluminum, silicon, phosphorous, sulfur, chlorine, argon, potassium, calcium, iron, copper, zinc, bromine, silver, iodine, gold, lead, mercury, radon. Day 3 99% of the atoms mass in the nucleus The energy of the atom in the electron shells Most of an atom empty space ...
... sodium, magnesium, aluminum, silicon, phosphorous, sulfur, chlorine, argon, potassium, calcium, iron, copper, zinc, bromine, silver, iodine, gold, lead, mercury, radon. Day 3 99% of the atoms mass in the nucleus The energy of the atom in the electron shells Most of an atom empty space ...
Chemistry 102B What`s in an atom? Before “Chemistry” Other Early
... Developed the “Law of conservation of Mass”. • Joseph Proust (early 1800s) – discovered that a given compound always contained the same proportions of certain elements by mass. “Law of Definite Proportions” • John Dalton (early 1800s) – noted that elements that combined to form more than one kind of ...
... Developed the “Law of conservation of Mass”. • Joseph Proust (early 1800s) – discovered that a given compound always contained the same proportions of certain elements by mass. “Law of Definite Proportions” • John Dalton (early 1800s) – noted that elements that combined to form more than one kind of ...
WHAT YOU NEED TO KNOW Electron Configurations Explain the
... Explain the relationship between energy levels and sublevels and atomic orbitals. Describe the shapes of the s & p orbitals. Recall the reason for the x, y, z, axes. Apply the Pauli exclusion principle, the aufbau principle, and Hund’s rule to write electron configurations using orbital diag ...
... Explain the relationship between energy levels and sublevels and atomic orbitals. Describe the shapes of the s & p orbitals. Recall the reason for the x, y, z, axes. Apply the Pauli exclusion principle, the aufbau principle, and Hund’s rule to write electron configurations using orbital diag ...
C2_Chemistry_Summary_Topic_1
... Keywords: proton, neutron, electron, shells, negative, atomic number, mass number ...
... Keywords: proton, neutron, electron, shells, negative, atomic number, mass number ...
Chapter 4 Study Guide Physical Science 1. The word atom comes
... 2. Halogens are very reactive elements located in Group _______of the periodic table. 3. The nucleus of an atom has a(n) ____________________ electric charge. 4. Carbon is found in group ______ of the periodic table. 5. Bohr’s model of the atom compares electrons to ____________________. 6. Elements ...
... 2. Halogens are very reactive elements located in Group _______of the periodic table. 3. The nucleus of an atom has a(n) ____________________ electric charge. 4. Carbon is found in group ______ of the periodic table. 5. Bohr’s model of the atom compares electrons to ____________________. 6. Elements ...
19. Define the law of multiple proportions. Elements form
... 19. Define the law of multiple proportions. Elements form compounds in simple whole number ratios 20. What do isotopes of the same element have in common and what is different? Isotopes have the same number of protons, electrons and atomic number Isotopes have a different mass number and number of n ...
... 19. Define the law of multiple proportions. Elements form compounds in simple whole number ratios 20. What do isotopes of the same element have in common and what is different? Isotopes have the same number of protons, electrons and atomic number Isotopes have a different mass number and number of n ...