Learning Standards vocab chemical basis and molecules of life 09
... two hydrogen atoms). Explain the meaning of a chemical formula for a molecule (e.g., CH4 or H2O).*a Demonstrate how carbon atoms form four covalent bonds to make large molecules. Identify the functions of these molecules (e.g., plant and animal tissue, polymers, sources of food and nutrition, fo ...
... two hydrogen atoms). Explain the meaning of a chemical formula for a molecule (e.g., CH4 or H2O).*a Demonstrate how carbon atoms form four covalent bonds to make large molecules. Identify the functions of these molecules (e.g., plant and animal tissue, polymers, sources of food and nutrition, fo ...
Quiz review
... Horizontal rows of the periodic table are called this. Vertical columns of the periodic table are called ‘groups’ or this. Which element in period 3 has 6 valence electrons? Which element in period 5 has only 1 electron in its 5s sublevel? Which element in period 3 has a full octet? What family of e ...
... Horizontal rows of the periodic table are called this. Vertical columns of the periodic table are called ‘groups’ or this. Which element in period 3 has 6 valence electrons? Which element in period 5 has only 1 electron in its 5s sublevel? Which element in period 3 has a full octet? What family of e ...
L.O.
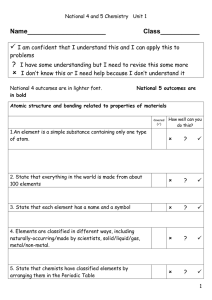
... I have some understanding but I need to revise this some more I don’t know this or I need help because I don’t understand it ...
... I have some understanding but I need to revise this some more I don’t know this or I need help because I don’t understand it ...
Chemistry Semester One Exam Review Name:
... 9. Given the electron configurations for the following neutral atoms, predict the oxidation number each is most likely to have. Element A B C D E ...
... 9. Given the electron configurations for the following neutral atoms, predict the oxidation number each is most likely to have. Element A B C D E ...
Chapter 6 Review“The Periodic Table”
... 1. How is the number of neutrons in the nucleus of an atom calculated? 2. All atoms are neutral, with the number of protons equaling the ___. 3. Isotopes of the same element have different _____. 4. Using the periodic table, determine the number of neutrons in 16O. 5. What does the number 84 represe ...
... 1. How is the number of neutrons in the nucleus of an atom calculated? 2. All atoms are neutral, with the number of protons equaling the ___. 3. Isotopes of the same element have different _____. 4. Using the periodic table, determine the number of neutrons in 16O. 5. What does the number 84 represe ...
– Units 5-7 Review Honors Chemistry Unit 5
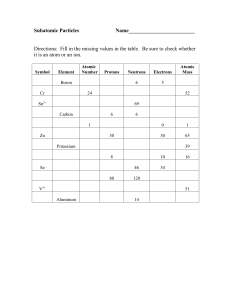
... How many protons, neutrons, and electrons are in Nickel-58? The element copper is found to contain the naturally occurring isotopes 29Cu63 and 29Cu65. The relative abundances are 69.1% and 30.9% respectively. Calculate the average atomic mass of copper. Ninety-two percent of the atoms of an element ...
... How many protons, neutrons, and electrons are in Nickel-58? The element copper is found to contain the naturally occurring isotopes 29Cu63 and 29Cu65. The relative abundances are 69.1% and 30.9% respectively. Calculate the average atomic mass of copper. Ninety-two percent of the atoms of an element ...
Atomic Crossword Name: Period: ____
... 11. A _____________ electron is in the outermost shell of an atom 12. Formed when atoms share electrons 14. Has a different number of neutrons than normal 15. The splitting of an atomic nucleus (usually with heavy elements) 17. British scientist who formulated our modern atomic theory 22. The mergin ...
... 11. A _____________ electron is in the outermost shell of an atom 12. Formed when atoms share electrons 14. Has a different number of neutrons than normal 15. The splitting of an atomic nucleus (usually with heavy elements) 17. British scientist who formulated our modern atomic theory 22. The mergin ...
here
... After reading these sections complete two of the following three options: 1. Explain what a valence electron is, and why it might make sense for valence electrons to be the “bonding/reactive” electrons. Then give an example of an element that would be likely to bond and one that would not be likely ...
... After reading these sections complete two of the following three options: 1. Explain what a valence electron is, and why it might make sense for valence electrons to be the “bonding/reactive” electrons. Then give an example of an element that would be likely to bond and one that would not be likely ...
Chapter 5
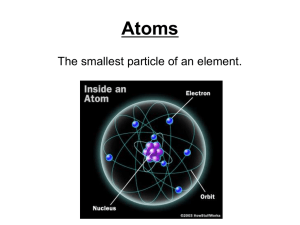
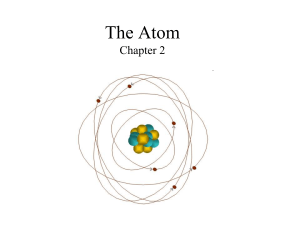
... •Electron cloud is 10,000 times larger than the nucleus, but is still mostly empty. •Electrons are in the cloud but can not be pinpointed at an exact time because they move so quickly. ...
... •Electron cloud is 10,000 times larger than the nucleus, but is still mostly empty. •Electrons are in the cloud but can not be pinpointed at an exact time because they move so quickly. ...
Ch. 4: Atoms and the Periodic Table – Study Guide
... to form a lithium ion with a charge of 1+. A lithium ion is much less reactive than a lithium atom because it has a full outermost energy level. Isotopes of an element have the same atomic number but different atomic mass. Group 1 of the periodic table consists of the alkali metals, a highly reactiv ...
... to form a lithium ion with a charge of 1+. A lithium ion is much less reactive than a lithium atom because it has a full outermost energy level. Isotopes of an element have the same atomic number but different atomic mass. Group 1 of the periodic table consists of the alkali metals, a highly reactiv ...
IPC Atoms and Periodic Table
... of the naturally occurring isotopes of an element • Reported as atomic mass on the periodic ...
... of the naturally occurring isotopes of an element • Reported as atomic mass on the periodic ...
CHE 1401 - Fall 2013 - Chapter 7 Homework 7 (Chapter 7: Periodic
... 1. It is not really a member of any particular group. 2. Its electron is not at all shielded from its nucleus. 3. It is the lightest element. 4. It is the only element to exist at room temperature as a diatomic gas. 5. It exhibits some chemical properties similar to those of groups 1A and 7A. A) 1, ...
... 1. It is not really a member of any particular group. 2. Its electron is not at all shielded from its nucleus. 3. It is the lightest element. 4. It is the only element to exist at room temperature as a diatomic gas. 5. It exhibits some chemical properties similar to those of groups 1A and 7A. A) 1, ...
CHEMISTRY TERMS Period: Elements in the same horizontal row
... Period: Elements in the same horizontal row with the same ground state energy level. Periodic Law: Elements list in order of their atomic numbers that fall into reoccurring groups. Ionic Radius: the radius of an atom’s ion, measured by the distance between ions in a crystal lattice. Atomic Radius: o ...
... Period: Elements in the same horizontal row with the same ground state energy level. Periodic Law: Elements list in order of their atomic numbers that fall into reoccurring groups. Ionic Radius: the radius of an atom’s ion, measured by the distance between ions in a crystal lattice. Atomic Radius: o ...
Chemical Basis of Life
... both hydrogen and oxygen are gases at room temperature 2 - Some compounds are quite simple others are very complex, the important thing to remember is that a compound is always in a fixed ratio ...
... both hydrogen and oxygen are gases at room temperature 2 - Some compounds are quite simple others are very complex, the important thing to remember is that a compound is always in a fixed ratio ...