The Chemical Basis of Life Chapter 4
... •Made of only one type of atom •Represented by 1-2 letters ...
... •Made of only one type of atom •Represented by 1-2 letters ...
Atoms
... Dmitri ________________________, a Russian scientist, arranged the elements into the Periodic Table. ...
... Dmitri ________________________, a Russian scientist, arranged the elements into the Periodic Table. ...
Atomic Structure and the Periodic Table
... • Electron cloud – cloud that surrounds the nucleus of an atom that describes the region in which an electron is most likely to be. – Example: students in a school ...
... • Electron cloud – cloud that surrounds the nucleus of an atom that describes the region in which an electron is most likely to be. – Example: students in a school ...
Basics of the Periodic Table
... *Directions: Use the Periodic Table in your textbook to answer the following questions. Read carefully! ...
... *Directions: Use the Periodic Table in your textbook to answer the following questions. Read carefully! ...
Properties of matter student notes[1]
... Element = Matter made of only _______________ kind of atom. Ex. Oxygen Can be naturally made or man-made. ...
... Element = Matter made of only _______________ kind of atom. Ex. Oxygen Can be naturally made or man-made. ...
Chemistry Ch 5-3 Notes: Periodic Trends
... increases as we move from left to right, because the larger number of protons holds onto electrons more strongly. The most difficult elements to remove electrons from are the noble gases (full valence) Also: The more electrons we try to remove, the harder it is to remove them, so first Ionization en ...
... increases as we move from left to right, because the larger number of protons holds onto electrons more strongly. The most difficult elements to remove electrons from are the noble gases (full valence) Also: The more electrons we try to remove, the harder it is to remove them, so first Ionization en ...
Physical Science Chapter 6 Study Guide Atomic Theory of Matter
... o Alpha decay—a nucleus emits a clump of two protons and two neutrons o Beta decay—a nucleus changes a neutron into a proton and ejects an electron o Gamma decay—a nucleus emits high-energy electromagnetic rays ...
... o Alpha decay—a nucleus emits a clump of two protons and two neutrons o Beta decay—a nucleus changes a neutron into a proton and ejects an electron o Gamma decay—a nucleus emits high-energy electromagnetic rays ...
What is Matter
... sharing electrons The first three periods of elements are the easiest to understand. The most eheld in the outer cloud of these elements is 8. In order to become more stable, elements will share e-. ...
... sharing electrons The first three periods of elements are the easiest to understand. The most eheld in the outer cloud of these elements is 8. In order to become more stable, elements will share e-. ...
HONORS CHEMISTRY Quarter 2 Exam Topics Know the following
... o Democritus, Dalton, Thomson, Rutherford, Chadwick, Bohr, Schrodinger, Heisenberg Know experimental observations and their key contributions to the atomic theory. Be able to draw and label the model of the atom for each scientist mentioned above. Know Dalton’s postulates. Distinguish atoms ba ...
... o Democritus, Dalton, Thomson, Rutherford, Chadwick, Bohr, Schrodinger, Heisenberg Know experimental observations and their key contributions to the atomic theory. Be able to draw and label the model of the atom for each scientist mentioned above. Know Dalton’s postulates. Distinguish atoms ba ...
Lecture 3
... Also on closer inspection of the different n levels, additional fine structure is observed within each n level and these are assigned different letters of the alphabet. ...
... Also on closer inspection of the different n levels, additional fine structure is observed within each n level and these are assigned different letters of the alphabet. ...
Chemistry Review: Antoine Lavoisier (1743
... there are 3 isotopes of hydrogen, with mass numbers of 1, 2 and 3. These atoms have different masses because they have different numbers of neutrons. However, since they have the same number of protons, they are the same element. Most elements have more than 1 naturally occurring isotope. However, t ...
... there are 3 isotopes of hydrogen, with mass numbers of 1, 2 and 3. These atoms have different masses because they have different numbers of neutrons. However, since they have the same number of protons, they are the same element. Most elements have more than 1 naturally occurring isotope. However, t ...
Atomic Theory - rlhonorschem4
... » 1.All matter is composed of extremely small particles called atoms. » 2. Atoms of a given element are identical in size, mass, and other properties; atoms of different elements differ in size, mass, and other properties.' » 3.Atoms cannot be subdivided, created or destroyed. » 4.Atoms of different ...
... » 1.All matter is composed of extremely small particles called atoms. » 2. Atoms of a given element are identical in size, mass, and other properties; atoms of different elements differ in size, mass, and other properties.' » 3.Atoms cannot be subdivided, created or destroyed. » 4.Atoms of different ...
Worksheet
... 15. __________ He used the term “atomos” to describe an indivisible part at the base of all matter. 16. __________ He is the Father of the Atomic Theory. 17. __________ He designed a mathematical equation for the model of the atom. 18. __________ He determined the charge of the electron. 19. _______ ...
... 15. __________ He used the term “atomos” to describe an indivisible part at the base of all matter. 16. __________ He is the Father of the Atomic Theory. 17. __________ He designed a mathematical equation for the model of the atom. 18. __________ He determined the charge of the electron. 19. _______ ...
CH 115 Fall 2014Exam I Review Brief overview of topics/concepts to
... What does it mean for something to be quantized? Four quantum numbers – Range Abbreviation What do they each determine What makes a set of quantum numbers invalid Know shapes of the s and p orbitals Electron configurations – Pauli exclusion principle Hund’s rule Aufbau principle Co ...
... What does it mean for something to be quantized? Four quantum numbers – Range Abbreviation What do they each determine What makes a set of quantum numbers invalid Know shapes of the s and p orbitals Electron configurations – Pauli exclusion principle Hund’s rule Aufbau principle Co ...
Chapter 1 D Study Guide
... 3. Electrons move around the nucleus in electron rings or shells or energy levels. 4. Atomic number is equal to the number of protons, and is unique to each element 5. The number of protons is equal to the number of electrons in a balanced atom, but not in an ION 6. The atomic mass (rounded off) is ...
... 3. Electrons move around the nucleus in electron rings or shells or energy levels. 4. Atomic number is equal to the number of protons, and is unique to each element 5. The number of protons is equal to the number of electrons in a balanced atom, but not in an ION 6. The atomic mass (rounded off) is ...
Minerals * Chemistry Review
... • The number of protons plus neutrons gives the atom its atomic mass • All atoms of a given element have the same number of protons ...
... • The number of protons plus neutrons gives the atom its atomic mass • All atoms of a given element have the same number of protons ...
C1a - Mr Corfe
... Chloride – bleaches litmas paper When above is reacted with water Element + water → Element hydroxide + hydrogen REACTIVITY SERIES Most reactive least reactive caesium Cs rubidium Rb potassium K sodium Na lithium Li calcium Ca magnesium Mg aluminium Al zinc Zn iron Fe Gold Au s ...
... Chloride – bleaches litmas paper When above is reacted with water Element + water → Element hydroxide + hydrogen REACTIVITY SERIES Most reactive least reactive caesium Cs rubidium Rb potassium K sodium Na lithium Li calcium Ca magnesium Mg aluminium Al zinc Zn iron Fe Gold Au s ...
Chapter 4 - Bakersfield College
... Isotopes: atoms with the same number of protons and electrons but different numbers of neutrons. ...
... Isotopes: atoms with the same number of protons and electrons but different numbers of neutrons. ...
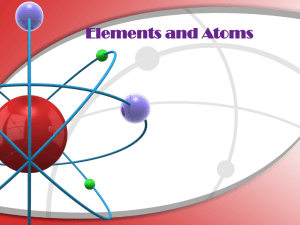
Elements and Atoms - Portola Middle School
... neutron or proton. Protons should have a + or P written on them. Neutrons should be blank or have an N. In a circle around the nucleus are the electrons. Electrons should have a minus sign or an e. ...
... neutron or proton. Protons should have a + or P written on them. Neutrons should be blank or have an N. In a circle around the nucleus are the electrons. Electrons should have a minus sign or an e. ...
Atomic Structure
... found in the __________ of the atom Neutrons – have __________charge and are also found in the __________of an atom Electrons – have a __________charge and are found __________ of the nucleus Nucleus – made up of __________and __________, has an overall __________ charge ...
... found in the __________ of the atom Neutrons – have __________charge and are also found in the __________of an atom Electrons – have a __________charge and are found __________ of the nucleus Nucleus – made up of __________and __________, has an overall __________ charge ...



![Properties of matter student notes[1]](http://s1.studyres.com/store/data/009076956_1-3293fc3fecf578fd34e3f0f2700d471f-300x300.png)