lecture_CH1-2review_chem121pikul
... Distinguish the difference between chemical and physical properties & changes We represent uncertainty with significant figures You do not need to memorize Sig Fig rules Scientific Notation Conversions within the metric system and non metric units Temperature conversions Density & Spec ...
... Distinguish the difference between chemical and physical properties & changes We represent uncertainty with significant figures You do not need to memorize Sig Fig rules Scientific Notation Conversions within the metric system and non metric units Temperature conversions Density & Spec ...
Chemical Change
... The chemical properties of elements are related to the energy changes that take place when atoms lose, gain or share electrons to obtain a filled valence shell. ...
... The chemical properties of elements are related to the energy changes that take place when atoms lose, gain or share electrons to obtain a filled valence shell. ...
AP Chem Test 5-7 Practice Exam - mvhs
... 2. The ΔH for the exothermic solution process when solid sodium hydroxide dissolves in water is 44.4 kJ/mol. When a 13.9-g sample of NaOH dissolves in 250.0 g of water in a coffee-cup calorimeter, the temperature change is _______. Assume that the solution has the same specific heat as liquid water, ...
... 2. The ΔH for the exothermic solution process when solid sodium hydroxide dissolves in water is 44.4 kJ/mol. When a 13.9-g sample of NaOH dissolves in 250.0 g of water in a coffee-cup calorimeter, the temperature change is _______. Assume that the solution has the same specific heat as liquid water, ...
Topic 4: Classifying Elements What did the early chemists use to
... • NH3(g) à nitrogen trihydride or ammonia • CH4(g) à carbon tetrahydride or methane • H2O2(l) à dihydrogen monoxide or water A MOLECULAR COMPOUND can contain what two combinations of elements? Non-‐metal + ...
... • NH3(g) à nitrogen trihydride or ammonia • CH4(g) à carbon tetrahydride or methane • H2O2(l) à dihydrogen monoxide or water A MOLECULAR COMPOUND can contain what two combinations of elements? Non-‐metal + ...
Atoms, Elements, Compounds File
... SOL 6.4 Atoms, Elements, compounds The student will investigate and understand that all matter is made up of atoms. Key concepts include ...
... SOL 6.4 Atoms, Elements, compounds The student will investigate and understand that all matter is made up of atoms. Key concepts include ...
1. Of the three major categories of elements (metals, non
... The name of the element, its chemical symbol, its atomic number and it average atomic mass. 11. What are the vertical columns called in the periodic table? They are called groups or families. 12. What are the horizontal rows on the periodic table called? They are called periods. 13. Explain the rela ...
... The name of the element, its chemical symbol, its atomic number and it average atomic mass. 11. What are the vertical columns called in the periodic table? They are called groups or families. 12. What are the horizontal rows on the periodic table called? They are called periods. 13. Explain the rela ...
1 - cloudfront.net
... How is the number of neutrons in the nucleus of an atom calculated? All atoms are neutral, with the number of protons equaling the ___. Isotopes of the same element have different _____. Using the periodic table, determine the number of neutrons in 16O. What does the number 84 represent in the name ...
... How is the number of neutrons in the nucleus of an atom calculated? All atoms are neutral, with the number of protons equaling the ___. Isotopes of the same element have different _____. Using the periodic table, determine the number of neutrons in 16O. What does the number 84 represent in the name ...
CH.2
... determine the atomic number, atomic mass, the number of protons, and the number of electrons of any atom of a particular element using a periodic table. (B5) determine the number of neutrons in an isotope given its mass number. (B5) perform calculations to determine the “weighted” average atomic ...
... determine the atomic number, atomic mass, the number of protons, and the number of electrons of any atom of a particular element using a periodic table. (B5) determine the number of neutrons in an isotope given its mass number. (B5) perform calculations to determine the “weighted” average atomic ...
Chapter 21 Powerpoint: Nuclear Chemistry
... After 10 half-lives sample considered nonradioactive because it approaches the level of background radiation. Because the amount never reaches zero, radioactive waste disposal and storage causes ...
... After 10 half-lives sample considered nonradioactive because it approaches the level of background radiation. Because the amount never reaches zero, radioactive waste disposal and storage causes ...
Chapter 5
... •Electron cloud is 10,000 times larger than the nucleus, but is still mostly empty. •Electrons are in the cloud but can not be pinpointed at an exact time because they move so quickly. ...
... •Electron cloud is 10,000 times larger than the nucleus, but is still mostly empty. •Electrons are in the cloud but can not be pinpointed at an exact time because they move so quickly. ...
Atom through Periodic Table Study Guide
... ____7. Determined the charge then calculated the mass of an electron in his oil drop experiment. ____8. Worked in Rutherford’s lab on the gold foil experiment, a graduate student who suggested that Rutherford should let Marsden get some lab experience. ____9. Believed that the world was made of mat ...
... ____7. Determined the charge then calculated the mass of an electron in his oil drop experiment. ____8. Worked in Rutherford’s lab on the gold foil experiment, a graduate student who suggested that Rutherford should let Marsden get some lab experience. ____9. Believed that the world was made of mat ...
Minerals * Chemistry Review
... • Protons have a positive charge and are located in the nucleus • Neutrons have no charge and are located in the nucleus • Electrons are negatively charged and are located outside of the nucleus ...
... • Protons have a positive charge and are located in the nucleus • Neutrons have no charge and are located in the nucleus • Electrons are negatively charged and are located outside of the nucleus ...
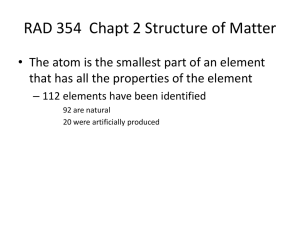
RAD 354 Chapt 3 Structure of Matter
... • The atom is the smallest part of an element that has all the properties of the element – 112 elements have been identified 92 are natural 20 were artificially produced ...
... • The atom is the smallest part of an element that has all the properties of the element – 112 elements have been identified 92 are natural 20 were artificially produced ...
Unit 1: Atomic Structure AP Chemistry
... experiment 1.60 x 10-19 From this and Thomson’s value, the mass was calculated to be 9.11 x 10-28g ...
... experiment 1.60 x 10-19 From this and Thomson’s value, the mass was calculated to be 9.11 x 10-28g ...
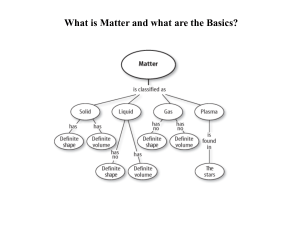
Matter and the Periodic Table
... system of rows and columns on the basis of increasing mass and similar chemical and physical properties. Since the organization exhibited a periodic repetition of similar properties, it became known as the Periodic Table of the Elements. It has become one of modern chemistry's ...
... system of rows and columns on the basis of increasing mass and similar chemical and physical properties. Since the organization exhibited a periodic repetition of similar properties, it became known as the Periodic Table of the Elements. It has become one of modern chemistry's ...
8.P.1.1 Warm-Up Questions for Website
... B.It can be formed through a physical reaction. C.It can be changed into simpler substances through a physical change. D.It is a pure substance containing elements that are chemically combined. ...
... B.It can be formed through a physical reaction. C.It can be changed into simpler substances through a physical change. D.It is a pure substance containing elements that are chemically combined. ...
Salesian High School Elements and atoms Chemistry quiz The
... 7) Which of the following is one of the statements that make up Dalton’s atomic theory? a) All atoms contain electrons. b) All atoms of a given element are identical. c) Atoms are divisible. d) Atoms gain and lose electrons in chemical reactions ...
... 7) Which of the following is one of the statements that make up Dalton’s atomic theory? a) All atoms contain electrons. b) All atoms of a given element are identical. c) Atoms are divisible. d) Atoms gain and lose electrons in chemical reactions ...
Radioisotopes
... * Atomic number = number of protons or electrons * Atomic mass = number of nucleons (protons + neutrons). ...
... * Atomic number = number of protons or electrons * Atomic mass = number of nucleons (protons + neutrons). ...
BC1 Atoms Unit Standards
... periodic table based on properties, number of protons, or electron configuration) Create visual models for atoms of different elements that shows the location of electrons and their relative distance from the nucleus. 4a Draw a Bohr model for a given element 4b Write an electron configuration to des ...
... periodic table based on properties, number of protons, or electron configuration) Create visual models for atoms of different elements that shows the location of electrons and their relative distance from the nucleus. 4a Draw a Bohr model for a given element 4b Write an electron configuration to des ...
Periodic Table of Elements * Study Guide
... Study and understand the following: Atomic Structure: How to find an element’s: atomic number atomic mass what two particles make up the atomic mass? what makes up the atom’s volume? # of protons Electrical charge of proton, electron, neutron # of electrons # of neutrons group # ...
... Study and understand the following: Atomic Structure: How to find an element’s: atomic number atomic mass what two particles make up the atomic mass? what makes up the atom’s volume? # of protons Electrical charge of proton, electron, neutron # of electrons # of neutrons group # ...
The Atom Chapter 2
... • Heteronuclear Diatomic Molecule: a molecule made of two atoms that are different elements – NO ...
... • Heteronuclear Diatomic Molecule: a molecule made of two atoms that are different elements – NO ...