Elements and Atoms - Portola Middle School
... neutron or proton. Protons should have a + or P written on them. Neutrons should be blank or have an N. In a circle around the nucleus are the electrons. Electrons should have a minus sign or an e. ...
... neutron or proton. Protons should have a + or P written on them. Neutrons should be blank or have an N. In a circle around the nucleus are the electrons. Electrons should have a minus sign or an e. ...
Periodic Table Review Key
... 1. Which element has the smallest atomic number? F 2. Which element has the largest atomic number? H 3. Which non-metal has the smallest atomic mass? F 4. Which metal has the largest atomic mass? D 5. Which elements are considered good conductors? C,E,D, 6. Which element is considered semi-conductor ...
... 1. Which element has the smallest atomic number? F 2. Which element has the largest atomic number? H 3. Which non-metal has the smallest atomic mass? F 4. Which metal has the largest atomic mass? D 5. Which elements are considered good conductors? C,E,D, 6. Which element is considered semi-conductor ...
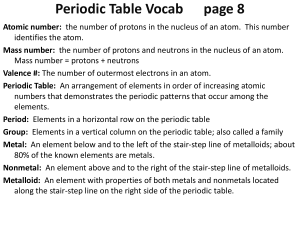
Periodic Table Vocab page 7
... Atomic number: the number of protons in the nucleus of an atom. This number identifies the atom. Mass number: the number of protons and neutrons in the nucleus of an atom. Mass number = protons + neutrons Valence #: The number of outermost electrons in an atom. Periodic Table: An arrangement of elem ...
... Atomic number: the number of protons in the nucleus of an atom. This number identifies the atom. Mass number: the number of protons and neutrons in the nucleus of an atom. Mass number = protons + neutrons Valence #: The number of outermost electrons in an atom. Periodic Table: An arrangement of elem ...
Atomic Structure and Nuclear Chemistry OEQs
... Do you believe that there are smaller subatomic particles than the ones that are currently believed to be the smallest? Explain. The Periodic Table of Elements is an organized table that contains all of the elements known-to-date. How the Periodic Table developed and what are its key features? ...
... Do you believe that there are smaller subatomic particles than the ones that are currently believed to be the smallest? Explain. The Periodic Table of Elements is an organized table that contains all of the elements known-to-date. How the Periodic Table developed and what are its key features? ...
What is the history of chemistry and elements
... 2. What is the structure of an atom? 3. How are ions formed from atoms? History 2400 year ago Greek philosophers proposed that everything was made of four basic substances – air, water, fire, and earth. Today chemists know that there are 100+ basic substances, or elements. Everything on Earth ...
... 2. What is the structure of an atom? 3. How are ions formed from atoms? History 2400 year ago Greek philosophers proposed that everything was made of four basic substances – air, water, fire, and earth. Today chemists know that there are 100+ basic substances, or elements. Everything on Earth ...
PS 2.2
... the weighted average of the masses of the naturally occurring isotopes of an element. The atomic mass of an element can be found on the periodic table. Since it is an average, it is usually not a whole number. ...
... the weighted average of the masses of the naturally occurring isotopes of an element. The atomic mass of an element can be found on the periodic table. Since it is an average, it is usually not a whole number. ...
Chemical Bonds
... compounds An atom is chemically stable when it has a complete outer energy level ...
... compounds An atom is chemically stable when it has a complete outer energy level ...
Chapter 6 Vocabulary crossword puzzle
... 3. Elements in which the highest occupied s and p sublevels are partially filled 6. Measures the ability of an atom to attract electrons when the atom is in a compound; the element named Cesium has the lowest amount, while the element named Fluorine has the highest amount 7. Term that refers to a se ...
... 3. Elements in which the highest occupied s and p sublevels are partially filled 6. Measures the ability of an atom to attract electrons when the atom is in a compound; the element named Cesium has the lowest amount, while the element named Fluorine has the highest amount 7. Term that refers to a se ...
Chem vocab quiz definitons
... Valence electrons are the electrons in the outer shell and control the elements reactivity. Molecule is the smallest particle of a compound that has all the properties of the compound. Compound is a pure substance that is made of two or more elements chemically bound together. Pure substances are bo ...
... Valence electrons are the electrons in the outer shell and control the elements reactivity. Molecule is the smallest particle of a compound that has all the properties of the compound. Compound is a pure substance that is made of two or more elements chemically bound together. Pure substances are bo ...
Introduction_to_Geochemistry_Pre-Lecture_Quiz
... detach the loosest electron from atoms of that element. (e) All alkali metals have similar chemical properties. (f) Alkali earths have one electron in the outer shell. (g) Electronegativity is the amount of negative charge on an atom. (h) Ca has a valency of 2. (i) True ionic bonds are unknown and a ...
... detach the loosest electron from atoms of that element. (e) All alkali metals have similar chemical properties. (f) Alkali earths have one electron in the outer shell. (g) Electronegativity is the amount of negative charge on an atom. (h) Ca has a valency of 2. (i) True ionic bonds are unknown and a ...
1.2 Atomic Theory
... The average atomic mass for magnesium found on the periodic table is a weighted average of the three isotopes: 24.31 g of Mg Radioactivity: spontaneous decay of nuclei, releasing energy and subatomic particles Radioisotopes: an unstable isotope of an element, which undergoes radioactive decay ...
... The average atomic mass for magnesium found on the periodic table is a weighted average of the three isotopes: 24.31 g of Mg Radioactivity: spontaneous decay of nuclei, releasing energy and subatomic particles Radioisotopes: an unstable isotope of an element, which undergoes radioactive decay ...
Chemistry Semester One Exam Review Name:
... 11. Write the electron configurations for the following elements. LithiumNitrogenZincBromineBarium12. What is the characteristic set of valence electrons for the following groups on the periodic table? Alkali metals (1); alkaline earth metals (2); halogens (17); noble gases (18) ...
... 11. Write the electron configurations for the following elements. LithiumNitrogenZincBromineBarium12. What is the characteristic set of valence electrons for the following groups on the periodic table? Alkali metals (1); alkaline earth metals (2); halogens (17); noble gases (18) ...
File
... composition that is uniform throughout, all the way down to the molecular level. • Hydrocarbon-any molecule consisting of only hydrogen and carbon atoms, typically fossil fuels and other compounds derived from them. • Ion- a charged atom, it has either gained or lost an electron. • Isotope-any varie ...
... composition that is uniform throughout, all the way down to the molecular level. • Hydrocarbon-any molecule consisting of only hydrogen and carbon atoms, typically fossil fuels and other compounds derived from them. • Ion- a charged atom, it has either gained or lost an electron. • Isotope-any varie ...
Intro to Atoms Clicker Questions 1. "atomos" means? 2. Atoms of one
... are located where? 6. Rutherford's proof of the proton's location in the atom came from an experiment with _______ 7. In the Bohr model of the atom, electrons are arranged how? 8. A neutron has (a) _____ charge 9. (T/F) The number of neutrons in an atom has to equal the number of protons. 10. (T/F) ...
... are located where? 6. Rutherford's proof of the proton's location in the atom came from an experiment with _______ 7. In the Bohr model of the atom, electrons are arranged how? 8. A neutron has (a) _____ charge 9. (T/F) The number of neutrons in an atom has to equal the number of protons. 10. (T/F) ...
Page 233 - ClassZone
... the world differed because each was made of atoms of different sizes and shapes. How does the modern view of atoms differ from this ancient view? How is it similar? ...
... the world differed because each was made of atoms of different sizes and shapes. How does the modern view of atoms differ from this ancient view? How is it similar? ...
The Chemical Basis of Life Chapter 4
... The Atom • The atom is the smallest part of an element that retains all the characteristics of that element. • A Greek scientist named Democritus was one of the first to propose that all matter was composed of tiny particles called atoms ...
... The Atom • The atom is the smallest part of an element that retains all the characteristics of that element. • A Greek scientist named Democritus was one of the first to propose that all matter was composed of tiny particles called atoms ...
Homework Geochem Test Review
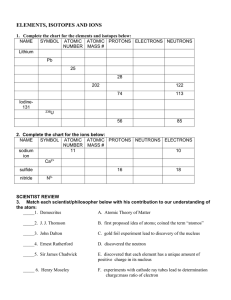
... 12. How many protons, neutrons and electrons are in the following elements? A) Fe ( iron) ...
... 12. How many protons, neutrons and electrons are in the following elements? A) Fe ( iron) ...
The study of biology can help you better understand human
... 10. Atomic mass is measured in ____________________________11. How do the isotopes of an element differ?_________________________________ How are they alike? ______________________________________________ 12. The number 37 in the name chlorine-37 represents __________________ 13. What does each numb ...
... 10. Atomic mass is measured in ____________________________11. How do the isotopes of an element differ?_________________________________ How are they alike? ______________________________________________ 12. The number 37 in the name chlorine-37 represents __________________ 13. What does each numb ...
Properties of matter student notes[1]
... Protons = _______________________ charged particles in the nucleus Neutrons = _____________________ particles in the nucleus ...
... Protons = _______________________ charged particles in the nucleus Neutrons = _____________________ particles in the nucleus ...
– Units 5-7 Review Honors Chemistry Unit 5
... How many protons, neutrons, and electrons are in Nickel-58? The element copper is found to contain the naturally occurring isotopes 29Cu63 and 29Cu65. The relative abundances are 69.1% and 30.9% respectively. Calculate the average atomic mass of copper. Ninety-two percent of the atoms of an element ...
... How many protons, neutrons, and electrons are in Nickel-58? The element copper is found to contain the naturally occurring isotopes 29Cu63 and 29Cu65. The relative abundances are 69.1% and 30.9% respectively. Calculate the average atomic mass of copper. Ninety-two percent of the atoms of an element ...

















![Properties of matter student notes[1]](http://s1.studyres.com/store/data/009076956_1-3293fc3fecf578fd34e3f0f2700d471f-300x300.png)
