element - Mrs. Phillips` Physical Science Webpage
... • The periodic table is arranged by increasing atomic number. – During Mendeleev’s time, this arrangement left several blanks, however, the table exhibited a regularly repeating pattern, which could be used to predict the properties of elements that had not been discovered yet. – He was proven right ...
... • The periodic table is arranged by increasing atomic number. – During Mendeleev’s time, this arrangement left several blanks, however, the table exhibited a regularly repeating pattern, which could be used to predict the properties of elements that had not been discovered yet. – He was proven right ...
Introduction to the Periodic Table
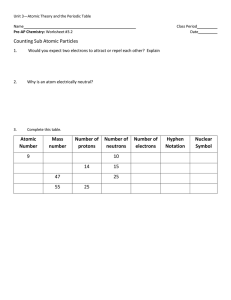
... The number of protons and neutrons in the nucleus of an atom. ...
... The number of protons and neutrons in the nucleus of an atom. ...
Name: Chapter 4 and 5 Study Guide Who was the Greek
... b. Its immediate acceptance by other scientists. c. The discovery of elements with predicted properties. d. The discovery of the nucleus 19. Moving from left to right across a row of the periodic table, which value increases by exactly one from element to element? ...
... b. Its immediate acceptance by other scientists. c. The discovery of elements with predicted properties. d. The discovery of the nucleus 19. Moving from left to right across a row of the periodic table, which value increases by exactly one from element to element? ...
Periodic Table
... –Included elements Valence # (bonding power) •How many e- the atom can lose or gain when bonding •Pattern appeared - 1234321 ...
... –Included elements Valence # (bonding power) •How many e- the atom can lose or gain when bonding •Pattern appeared - 1234321 ...
Chemistry Notes
... sodium, magnesium, aluminum, silicon, phosphorous, sulfur, chlorine, argon, potassium, calcium, iron, copper, zinc, bromine, silver, iodine, gold, lead, mercury, radon. Day 3 99% of the atoms mass in the nucleus The energy of the atom in the electron shells Most of an atom empty space ...
... sodium, magnesium, aluminum, silicon, phosphorous, sulfur, chlorine, argon, potassium, calcium, iron, copper, zinc, bromine, silver, iodine, gold, lead, mercury, radon. Day 3 99% of the atoms mass in the nucleus The energy of the atom in the electron shells Most of an atom empty space ...
File
... b. How do you know? _____________________________________________________________________ c. Identify the solute: _____________________________________________________________________ d. Identify the solvent: ____________________________________________________________________ 1. List the 4 signs of ...
... b. How do you know? _____________________________________________________________________ c. Identify the solute: _____________________________________________________________________ d. Identify the solvent: ____________________________________________________________________ 1. List the 4 signs of ...
Learning Objectives
... Atoms and Molecules 3. Draw and label a simplified model of an atom. Explain how this model simplifies our understanding of atomic structure. 4. Distinguish between each of the following pairs of terms: a. neutron and proton b. atomic number and mass number c. atomic weight and mass number 5. Explai ...
... Atoms and Molecules 3. Draw and label a simplified model of an atom. Explain how this model simplifies our understanding of atomic structure. 4. Distinguish between each of the following pairs of terms: a. neutron and proton b. atomic number and mass number c. atomic weight and mass number 5. Explai ...
Chemistry Overview
... • Element: pure substance that can’t be broken down into simpler substances • Element Symbols – 1-3 letters – Begin with 1 capital letter – Some based on Latin names • Ex/ gold = Au for Aurum iron = Fe for Ferros ...
... • Element: pure substance that can’t be broken down into simpler substances • Element Symbols – 1-3 letters – Begin with 1 capital letter – Some based on Latin names • Ex/ gold = Au for Aurum iron = Fe for Ferros ...
Chemistry Unit Study Guide Key
... 8) how to find elements on the periodic table – You can use the group and period that the element is in, it’s atomic number, or it’s atomic mass. 9) examples of heterogeneous and homogeneous mixtures – heterogeneous: ...
... 8) how to find elements on the periodic table – You can use the group and period that the element is in, it’s atomic number, or it’s atomic mass. 9) examples of heterogeneous and homogeneous mixtures – heterogeneous: ...
Understanding the Atom GN
... When atoms of the same element have different numbers of neutrons they are called ____________________. Isotope – ________________________________________________________________________ Most elements have ______________________ isotopes. Mass Number - ________________________________________ ...
... When atoms of the same element have different numbers of neutrons they are called ____________________. Isotope – ________________________________________________________________________ Most elements have ______________________ isotopes. Mass Number - ________________________________________ ...
2.1 The Nature of Matter - Sonoma Valley High School
... Some elements have isotopes, with different #s of neutrons and different mass. All isotopes of an element have the same chemical properties b/c their electrons are the same. ...
... Some elements have isotopes, with different #s of neutrons and different mass. All isotopes of an element have the same chemical properties b/c their electrons are the same. ...
Chapter 11 and 12-2 Review/Study Guide for Test
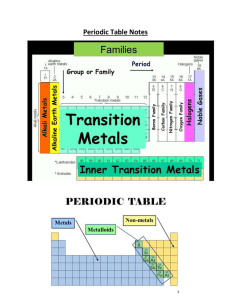
... 13. Explain the difference between a group and a period on the periodic table of elements. Groups = columns (there are 18 total) and Periods = Rows (there are 7). Also, a group shares similar properties. 14. Why are neither the alkali metals nor the alkaline-earth metals found uncombined in nature? ...
... 13. Explain the difference between a group and a period on the periodic table of elements. Groups = columns (there are 18 total) and Periods = Rows (there are 7). Also, a group shares similar properties. 14. Why are neither the alkali metals nor the alkaline-earth metals found uncombined in nature? ...
Review Notes - Biochemistry
... 5. Chemical Formula: Where each _ELEMENT_ is represented by its chemical _SYMBOL_ and the _NUMBER__ of atoms is shown in __SUBSCRIPTS__. ...
... 5. Chemical Formula: Where each _ELEMENT_ is represented by its chemical _SYMBOL_ and the _NUMBER__ of atoms is shown in __SUBSCRIPTS__. ...
Chemistry 102B What`s in an atom? Before “Chemistry” Other Early
... Before “Chemistry” • Alchemy/Alchemists - a pseudoscience built around trying to turn cheap metals into GOLD! (400 B.C.-1400 A.D.) • Metallurgy – systematic extraction of metals from ores laid some groundwork for modern chemistry. (1500s) • The first “chemist” was Robert Boyle who worked on pressure ...
... Before “Chemistry” • Alchemy/Alchemists - a pseudoscience built around trying to turn cheap metals into GOLD! (400 B.C.-1400 A.D.) • Metallurgy – systematic extraction of metals from ores laid some groundwork for modern chemistry. (1500s) • The first “chemist” was Robert Boyle who worked on pressure ...
Extra Credit Test Review
... 11.How would you write a lithium atom isotope with 5 neutrons? ___________ 12.One atom has 17 protons, 18 neutrons, and 17 electrons. Another atom has 17 protons, 19 neutrons and 17 electrons. Are these the same element? Yes No Explain: _______________________________________________________________ ...
... 11.How would you write a lithium atom isotope with 5 neutrons? ___________ 12.One atom has 17 protons, 18 neutrons, and 17 electrons. Another atom has 17 protons, 19 neutrons and 17 electrons. Are these the same element? Yes No Explain: _______________________________________________________________ ...
Period Table, valence Electrons and Ion Notes
... Example: Na = 1s2 2s2 2p6 3s1 Add up the e-‘s found in the last energy level, in this case there is only 1 so Na has 1 valence e**You have to do this for the Transition metal every time** ...
... Example: Na = 1s2 2s2 2p6 3s1 Add up the e-‘s found in the last energy level, in this case there is only 1 so Na has 1 valence e**You have to do this for the Transition metal every time** ...
Chemical reactions revision
... Mixtures can be separated more easily than compounds The technique used depends on the properties of the substances Iron can be separated by sulphur by a magnet. Some liquids can be separated by differences in their boiling points This is distillation ...
... Mixtures can be separated more easily than compounds The technique used depends on the properties of the substances Iron can be separated by sulphur by a magnet. Some liquids can be separated by differences in their boiling points This is distillation ...