Periodic Scavenger Hunt - bates
... 8. The atomic mass of an element is a combination of the number of protons and neutrons. Because the same element does not always have the same number of neutrons, the atomic mass is an average mass of the element as it occurs in nature. What is the atomic mass of fluorine? ...
... 8. The atomic mass of an element is a combination of the number of protons and neutrons. Because the same element does not always have the same number of neutrons, the atomic mass is an average mass of the element as it occurs in nature. What is the atomic mass of fluorine? ...
Lecture 3
... Isotopes are atoms of the same element that have different mass numbers; they have the same number of protons but different numbers of neutrons Isotopes are difficult to separate from each other, therefore they occur in any sample of the element in their natural abundance which remains relatively co ...
... Isotopes are atoms of the same element that have different mass numbers; they have the same number of protons but different numbers of neutrons Isotopes are difficult to separate from each other, therefore they occur in any sample of the element in their natural abundance which remains relatively co ...
T1 Final Study Guide - District 196 e
... a. The basketball is orange Intensive b. The diameter of the basketball is 31 centimeters Extensive c. The surface of the basketball has indented seams. Intensive d. The density of copper is 8.92 g/cm3. Intensive 7. Determine the number of significant figures in each of the following measurement res ...
... a. The basketball is orange Intensive b. The diameter of the basketball is 31 centimeters Extensive c. The surface of the basketball has indented seams. Intensive d. The density of copper is 8.92 g/cm3. Intensive 7. Determine the number of significant figures in each of the following measurement res ...
The Nature of Molecules
... nucleus of an atom; one level contains only 1 orbit of electrons, others contain 4 different orbits of electrons (each orbit is filled with 2 e-’s) • The filling of orbitals and energy levels relates to the chemical behavior of atoms • The number of electrons of an atom relates to its valence • Vale ...
... nucleus of an atom; one level contains only 1 orbit of electrons, others contain 4 different orbits of electrons (each orbit is filled with 2 e-’s) • The filling of orbitals and energy levels relates to the chemical behavior of atoms • The number of electrons of an atom relates to its valence • Vale ...
TERM 2 Unit 3 YR 9 SCI It is elementary
... understandings of atomic structure. Students model an atom according to currently accepted understandings. They will identify patterns in atomic structure that allow prediction of the products of chemical reactions and are reflected by the periodic table. They recognise that new substances are forme ...
... understandings of atomic structure. Students model an atom according to currently accepted understandings. They will identify patterns in atomic structure that allow prediction of the products of chemical reactions and are reflected by the periodic table. They recognise that new substances are forme ...
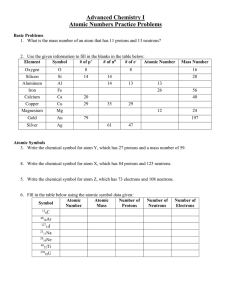
Atomic Numbers Practice Problems
... 3. Write the chemical symbol for atom Y, which has 27 protons and a mass number of 59. ...
... 3. Write the chemical symbol for atom Y, which has 27 protons and a mass number of 59. ...
Periodic Table ppt
... atom is also the Atomic number, so therefore the Atomic number also represents the amount of Protons in the nucleus of that Atom. ...
... atom is also the Atomic number, so therefore the Atomic number also represents the amount of Protons in the nucleus of that Atom. ...
2.2 Periodic Trends
... What are the trends that occur in the periodic table by organizing elements by their atomic number? Periodic trends are specific patterns that are present in the periodic table that illustrate different aspects of a certain element. Periodic trends, arising from the arrangement of the periodic t ...
... What are the trends that occur in the periodic table by organizing elements by their atomic number? Periodic trends are specific patterns that are present in the periodic table that illustrate different aspects of a certain element. Periodic trends, arising from the arrangement of the periodic t ...
AP Chapter 2 Objectives
... Give the approximate size, relative mass, and charge of an atom, proton, neutron, and electron Distinguish between physical and chemical properties and also physical and chemical changes. ...
... Give the approximate size, relative mass, and charge of an atom, proton, neutron, and electron Distinguish between physical and chemical properties and also physical and chemical changes. ...
Ch 3 studentElements Ions Isotopes
... 2. all atoms of a particular element are identical 3. different elements have different atoms 4. atoms combine in certain whole-number ratios 5. In a chemical reaction, atoms are merely rearranged to form new compounds; they are not created, destroyed, or changed into atoms of any other elements ...
... 2. all atoms of a particular element are identical 3. different elements have different atoms 4. atoms combine in certain whole-number ratios 5. In a chemical reaction, atoms are merely rearranged to form new compounds; they are not created, destroyed, or changed into atoms of any other elements ...
levels of organization and the atom
... It is the basic unit of matter. Atomos “means unable to cut”. It has two areas: a nucleus and an electron cloud. It contains subatomic particles that are even smaller. ...
... It is the basic unit of matter. Atomos “means unable to cut”. It has two areas: a nucleus and an electron cloud. It contains subatomic particles that are even smaller. ...
File
... Wavelengths corresponding to light given out when electrons moved from a higher to lower shell. 9. What is the outer shell of electrons called? ...
... Wavelengths corresponding to light given out when electrons moved from a higher to lower shell. 9. What is the outer shell of electrons called? ...
04 Atoms_ molecules _ ions
... •Left three quarters of the chart •Lose electrons •Become positive ...
... •Left three quarters of the chart •Lose electrons •Become positive ...
Introduction to Atomic Theory
... 2. Atoms of the same element are identical. The atoms of any one element are different from those of any other element. 3. Atoms of different elements can combine with one another in single whole number ratios to form compounds. 4. Chemical reactions occur when atoms are separated, joined, or rearra ...
... 2. Atoms of the same element are identical. The atoms of any one element are different from those of any other element. 3. Atoms of different elements can combine with one another in single whole number ratios to form compounds. 4. Chemical reactions occur when atoms are separated, joined, or rearra ...
Atomic Timeline
... the path of electrons (orbits) around the positive nucleus ► These orbits are specific distances from the nucleus ► Electron energy level model. ...
... the path of electrons (orbits) around the positive nucleus ► These orbits are specific distances from the nucleus ► Electron energy level model. ...
Timeline Assignment
... Early Greek philosophers believed all matter was made up of four “elements” earth, air, water, and fire ...
... Early Greek philosophers believed all matter was made up of four “elements” earth, air, water, and fire ...
CHAPTER 1 Practice Exercises 1.1 12.3 g Cd 1.3 26.9814 u 1.5
... Conservation of mass derives from the postulate that atoms are not destroyed in chemical reactions. The Law of Definite Proportions derives from the notion that compounds are always composed of the same types and numbers of atoms of the various elements in the compound. ...
... Conservation of mass derives from the postulate that atoms are not destroyed in chemical reactions. The Law of Definite Proportions derives from the notion that compounds are always composed of the same types and numbers of atoms of the various elements in the compound. ...
The Atom - Williamstown Independent Schools
... Atoms cannot be subdivided, created or destroyed Atoms of different elements combine in simple, whole number ratios to form chemical compounds. In chemical reactions, atoms are combined, separated or rearranged. ...
... Atoms cannot be subdivided, created or destroyed Atoms of different elements combine in simple, whole number ratios to form chemical compounds. In chemical reactions, atoms are combined, separated or rearranged. ...
Atomic Structure and Periodic Table Review Guide
... 1.1 Atoms are the smallest form of elements Answer each question. You may use your book, reading guides, or reinforcement guides to help you. Answers do not have to be in complete sentences. 1. What does the atomic number tell you? 2. Where are electrons located in an atom and what is their charge? ...
... 1.1 Atoms are the smallest form of elements Answer each question. You may use your book, reading guides, or reinforcement guides to help you. Answers do not have to be in complete sentences. 1. What does the atomic number tell you? 2. Where are electrons located in an atom and what is their charge? ...
Physical Science Chapter 6 Study Guide Atomic Theory of Matter
... o All atoms of a particular element have the same properties o Different elements have different properties because their atoms are different o Atoms of different elements can combine in specific ways to form compounds o Chemical processes are the result of the rearrangement, combination, or separat ...
... o All atoms of a particular element have the same properties o Different elements have different properties because their atoms are different o Atoms of different elements can combine in specific ways to form compounds o Chemical processes are the result of the rearrangement, combination, or separat ...
C1a - Mr Corfe
... RULE: An metal is more reactive if it is further to the left of the periodic table or further down in the group (not including groups 3-8) TYPES OF REACTIONS PHYSICAL – changing of states EXOTHERMIC – gives out heat ENDOTHERMIC – take in heat from it surrounding THERMAL DECOMPOSITION – is a chemical ...
... RULE: An metal is more reactive if it is further to the left of the periodic table or further down in the group (not including groups 3-8) TYPES OF REACTIONS PHYSICAL – changing of states EXOTHERMIC – gives out heat ENDOTHERMIC – take in heat from it surrounding THERMAL DECOMPOSITION – is a chemical ...
Chapter 5 - Effingham County Schools
... nucleus, called the atomic number. For example, a hydrogen atom has 1 proton so its atomic number is 1. The total number of protons and neutrons in an atom’s nucleus is called its atomic mass number. Isotopes are atoms of the same element that have a different number of neutrons. Ions are formed whe ...
... nucleus, called the atomic number. For example, a hydrogen atom has 1 proton so its atomic number is 1. The total number of protons and neutrons in an atom’s nucleus is called its atomic mass number. Isotopes are atoms of the same element that have a different number of neutrons. Ions are formed whe ...