Slide 1
... We cannot show videos due to copyright. Sources for appropriate videos may include: Discovery Education and AGC Educational Media ...
... We cannot show videos due to copyright. Sources for appropriate videos may include: Discovery Education and AGC Educational Media ...
Ions and isotopes
... has a different number of neutrons than “normal” • Carbon has three isotopes ...
... has a different number of neutrons than “normal” • Carbon has three isotopes ...
L.O.
... 14. State that atoms of different elements are different and have a different number on the Periodic Table called the atomic number state that atoms of different elements have a different number of protons, called the atomic number ...
... 14. State that atoms of different elements are different and have a different number on the Periodic Table called the atomic number state that atoms of different elements have a different number of protons, called the atomic number ...
Quiz review
... Horizontal rows of the periodic table are called this. Vertical columns of the periodic table are called ‘groups’ or this. Which element in period 3 has 6 valence electrons? Which element in period 5 has only 1 electron in its 5s sublevel? Which element in period 3 has a full octet? What family of e ...
... Horizontal rows of the periodic table are called this. Vertical columns of the periodic table are called ‘groups’ or this. Which element in period 3 has 6 valence electrons? Which element in period 5 has only 1 electron in its 5s sublevel? Which element in period 3 has a full octet? What family of e ...
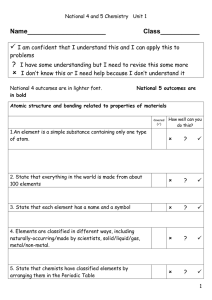
Atomic Crossword Name: Period: ____
... 11. A _____________ electron is in the outermost shell of an atom 12. Formed when atoms share electrons 14. Has a different number of neutrons than normal 15. The splitting of an atomic nucleus (usually with heavy elements) 17. British scientist who formulated our modern atomic theory 22. The mergin ...
... 11. A _____________ electron is in the outermost shell of an atom 12. Formed when atoms share electrons 14. Has a different number of neutrons than normal 15. The splitting of an atomic nucleus (usually with heavy elements) 17. British scientist who formulated our modern atomic theory 22. The mergin ...
Chapter 6 Review“The Periodic Table”
... Review“The Periodic Table” 1. How is the number of neutrons in the nucleus of an atom calculated? 2. All atoms are neutral, with the number of protons equaling the ___. 3. Isotopes of the same element have different _____. 4. Using the periodic table, determine the number of neutrons in 16O. 5. What ...
... Review“The Periodic Table” 1. How is the number of neutrons in the nucleus of an atom calculated? 2. All atoms are neutral, with the number of protons equaling the ___. 3. Isotopes of the same element have different _____. 4. Using the periodic table, determine the number of neutrons in 16O. 5. What ...
Chapter 4 Study Guide Physical Science 1. The word atom comes
... 1. The word atom comes from a Greek word that means “unable to be ____________________.” 2. Halogens are very reactive elements located in Group _______of the periodic table. 3. The nucleus of an atom has a(n) ____________________ electric charge. 4. Carbon is found in group ______ of the periodic t ...
... 1. The word atom comes from a Greek word that means “unable to be ____________________.” 2. Halogens are very reactive elements located in Group _______of the periodic table. 3. The nucleus of an atom has a(n) ____________________ electric charge. 4. Carbon is found in group ______ of the periodic t ...
File
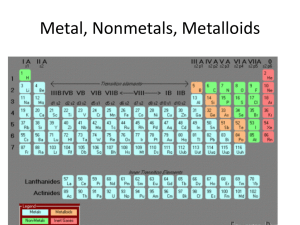
... element: a substance that cannot be separated or broken down into simpler substances by chemical means. compound: a substance made up of atoms of two ore more different elements joined by chemical bonds. atomic number: the number of protons in the nucleus of an atom. (The atomic number is the same f ...
... element: a substance that cannot be separated or broken down into simpler substances by chemical means. compound: a substance made up of atoms of two ore more different elements joined by chemical bonds. atomic number: the number of protons in the nucleus of an atom. (The atomic number is the same f ...
Summative Assessment Study Guide Name: Due date: SPS1
... Summative Assessment Study Guide Name: __________________________________Due date: _____________________ SPS1. Students will investigate our current understanding of the atom. a. Examine the structure of the atom in terms of ...
... Summative Assessment Study Guide Name: __________________________________Due date: _____________________ SPS1. Students will investigate our current understanding of the atom. a. Examine the structure of the atom in terms of ...
Chapter 14 Review
... 9. What information can be obtained by knowing the atomic number of an element? ...
... 9. What information can be obtained by knowing the atomic number of an element? ...
Periodic Table Puzzle
... The relative atomic mass of C is greater than the relative atomic mass of N but less than that of E. ...
... The relative atomic mass of C is greater than the relative atomic mass of N but less than that of E. ...
Chemical Basis of Life
... 1- Also for example water is a liquid at room temperature, whereas both hydrogen and oxygen are gases at room temperature 2 - Some compounds are quite simple others are very complex, the important thing to remember is that a compound is always in a fixed ratio ...
... 1- Also for example water is a liquid at room temperature, whereas both hydrogen and oxygen are gases at room temperature 2 - Some compounds are quite simple others are very complex, the important thing to remember is that a compound is always in a fixed ratio ...