Quiz 7 Name: 1. After ATP fuels the Na+/K+ pump at the cell
... C) NADH has more energy than NAD+. D) NADH can transfer electrons into the mitochondrial electron transport chain. 8. Cellular respiration harvests the most chemical energy from which of the following? A) glycolysis B) fermentation C) generating carbon dioxide and oxygen in the mitochondrial electro ...
... C) NADH has more energy than NAD+. D) NADH can transfer electrons into the mitochondrial electron transport chain. 8. Cellular respiration harvests the most chemical energy from which of the following? A) glycolysis B) fermentation C) generating carbon dioxide and oxygen in the mitochondrial electro ...
Aerobic Respiration - Weber State University
... oxidized to CO2. It is a cyclic process, with the pyruvate carbons getting attached to an existing cycle molecule prior to oxidation. Once the pyruvate carbons are completely oxidized, the cycle molecule needs to be regenerated. A little bit of ATP is made during the Krebs cycle, but mostly it is an ...
... oxidized to CO2. It is a cyclic process, with the pyruvate carbons getting attached to an existing cycle molecule prior to oxidation. Once the pyruvate carbons are completely oxidized, the cycle molecule needs to be regenerated. A little bit of ATP is made during the Krebs cycle, but mostly it is an ...
File
... • NADH and FADH2 are oxidized and O2 is reduced to H2O in a series of steps. • Series of redox carrier proteins embedded in the inner mitochondrial membrane. ...
... • NADH and FADH2 are oxidized and O2 is reduced to H2O in a series of steps. • Series of redox carrier proteins embedded in the inner mitochondrial membrane. ...
electron transport chain
... DG can be even higher than this in a cell This large amount of energy must be released in small steps rather than all at once. ...
... DG can be even higher than this in a cell This large amount of energy must be released in small steps rather than all at once. ...
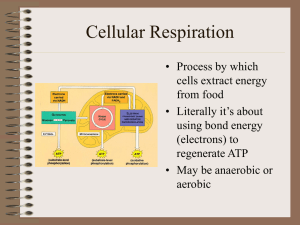
Cellular Respiration
... phosphorylation at the ETC? • The electrons, originally from glucose, are delivered to the ETC by NADH and FADH2, and are passed down the ETC. • This “electrical energy” runs a molecular machinery that pumps protons across the cristae. • These protons pass back through the cristae at the ATP synthas ...
... phosphorylation at the ETC? • The electrons, originally from glucose, are delivered to the ETC by NADH and FADH2, and are passed down the ETC. • This “electrical energy” runs a molecular machinery that pumps protons across the cristae. • These protons pass back through the cristae at the ATP synthas ...
SCI_7726_files/Cellular Respiration
... phosphorylation at the ETC? • The electrons, originally from glucose, are delivered to the ETC by NADH and FADH2, and are passed down the ETC. • This “electrical energy” runs a molecular machinery that pumps protons across the cristae. • These protons pass back through the cristae at the ATP synthas ...
... phosphorylation at the ETC? • The electrons, originally from glucose, are delivered to the ETC by NADH and FADH2, and are passed down the ETC. • This “electrical energy” runs a molecular machinery that pumps protons across the cristae. • These protons pass back through the cristae at the ATP synthas ...
How Cells Harvest Energy
... transferring a phosphate directly to ADP from another molecule 2. oxidative phosphorylation – use of ATP synthase and energy derived from a proton (H+) gradient to make ATP ...
... transferring a phosphate directly to ADP from another molecule 2. oxidative phosphorylation – use of ATP synthase and energy derived from a proton (H+) gradient to make ATP ...
The Process of Cellular Respiration
... • Chemiosmosis: an energy-coupling mechanism that uses energy stored in the form of an H+ gradient across a membrane to drive cellular work – In this case: coupling of the redox reactions of the electron transport chain to ATP synthesis ...
... • Chemiosmosis: an energy-coupling mechanism that uses energy stored in the form of an H+ gradient across a membrane to drive cellular work – In this case: coupling of the redox reactions of the electron transport chain to ATP synthesis ...
Bacterial Metabolism and Growth
... – All NADH molecules formed in the previous steps bring the electrons they have gained to the electron ...
... – All NADH molecules formed in the previous steps bring the electrons they have gained to the electron ...
electron transport chain
... The most active NADH shuttle, which functions in liver, kidney, and heart mitochondria, is the malate-aspartate ...
... The most active NADH shuttle, which functions in liver, kidney, and heart mitochondria, is the malate-aspartate ...
Respiration, Chapter 8
... molecules ( NADH & FADH2) down to oxygen Chemiosmosis: energy coupling mechanism ATP synthase: produces ATP by using the H+ gradient (proton-motive force) pumped into the inner membrane space from the electron transport chain; this enzyme harnesses the flow of H+ back into the matrix to phosphorylat ...
... molecules ( NADH & FADH2) down to oxygen Chemiosmosis: energy coupling mechanism ATP synthase: produces ATP by using the H+ gradient (proton-motive force) pumped into the inner membrane space from the electron transport chain; this enzyme harnesses the flow of H+ back into the matrix to phosphorylat ...
Ubiquinone
... • Chemicals like DNP and FCCP are weak acid with hydrophobic properties that permit them to diffuse readily across mitochondrial membranes. After entering the matrix in the protonated form, they can release a proton, thus disspating the proton gradient. ...
... • Chemicals like DNP and FCCP are weak acid with hydrophobic properties that permit them to diffuse readily across mitochondrial membranes. After entering the matrix in the protonated form, they can release a proton, thus disspating the proton gradient. ...
Exam #2
... Fermentation as a fate of pyruvate; endogenous organic electron acceptor; why does NADH need to be oxidized; how much energy does fermentation of glucose produce compared to aerobic respiration? What are some fermentation products other than lactic acid and ethanol? Krebs Cycle: what’s the role of t ...
... Fermentation as a fate of pyruvate; endogenous organic electron acceptor; why does NADH need to be oxidized; how much energy does fermentation of glucose produce compared to aerobic respiration? What are some fermentation products other than lactic acid and ethanol? Krebs Cycle: what’s the role of t ...
Electron transport chains in mitochondria
... The electron transport chain comprises an enzymatic series of electron donors and acceptors. Each electron donor passes electrons to a more electronegative acceptor, which in turn donates these electrons to another acceptor, a process that continues down the series until electrons are passed to oxyg ...
... The electron transport chain comprises an enzymatic series of electron donors and acceptors. Each electron donor passes electrons to a more electronegative acceptor, which in turn donates these electrons to another acceptor, a process that continues down the series until electrons are passed to oxyg ...
Chap 5
... 3. Glycolysis or the EMP pathway results in the breakdown of glucose to 2 pyruvate molecules (Fig.5.4, p.133) (1) under anaerobic conditions, pyruvate may be converted to lactic acid, ethanol, acetone, butanol, and acetic acid (2) under aerobic conditions, pyruvate is converted to CO2 and NADH throu ...
... 3. Glycolysis or the EMP pathway results in the breakdown of glucose to 2 pyruvate molecules (Fig.5.4, p.133) (1) under anaerobic conditions, pyruvate may be converted to lactic acid, ethanol, acetone, butanol, and acetic acid (2) under aerobic conditions, pyruvate is converted to CO2 and NADH throu ...
Study Guide - PEP 535 Exam#1
... What are the sources of protons during muscle contraction? What are the sources of proton buffering/utilization/removal in skeletal muscle? Is it correct to interpret lactate production as the cause of muscle acidosis? Why? Why does ATP hydrolysis release a proton? How would you explain the biochemi ...
... What are the sources of protons during muscle contraction? What are the sources of proton buffering/utilization/removal in skeletal muscle? Is it correct to interpret lactate production as the cause of muscle acidosis? Why? Why does ATP hydrolysis release a proton? How would you explain the biochemi ...
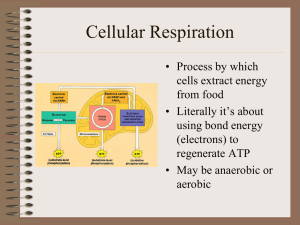
Cellular Respiration
... - does not require O2 ; occurs in cytoplasm Pyruvate Oxidation: chemical pathway that connects glycolysis to Krebs cycle 2 pyruvate molecules are moved from the cytoplasm to the matrix of the mitochondria CO2 is removed from each pyruvate molecule and released as a waste product (1/3 of what y ...
... - does not require O2 ; occurs in cytoplasm Pyruvate Oxidation: chemical pathway that connects glycolysis to Krebs cycle 2 pyruvate molecules are moved from the cytoplasm to the matrix of the mitochondria CO2 is removed from each pyruvate molecule and released as a waste product (1/3 of what y ...
cyt c - mustafaaltinisik.org.uk
... • Electrons from cyt c are used in a fourelectron reduction of O2 to produce 2H2O • Oxygen is thus the terminal acceptor of electrons in the electron transport pathway the end! • Cytochrome c oxidase utilizes 2 hemes (a and a3) and 2 copper sites • Complex IV also transports H+ (2 protons) ...
... • Electrons from cyt c are used in a fourelectron reduction of O2 to produce 2H2O • Oxygen is thus the terminal acceptor of electrons in the electron transport pathway the end! • Cytochrome c oxidase utilizes 2 hemes (a and a3) and 2 copper sites • Complex IV also transports H+ (2 protons) ...
Chapter 5 Test Review
... 6. enter the electron transport chain of the thylakoid membrane 7. water is split to replace 2e- that enter the electron transport chain of the thylakoid membrane, the O2 is released and the H+ join with NADP+ 8. PSII – ATP, PSI – NADPH 9. ATP (to Calvin cycle), NADPH (to Calvin Cycle), O2 (released ...
... 6. enter the electron transport chain of the thylakoid membrane 7. water is split to replace 2e- that enter the electron transport chain of the thylakoid membrane, the O2 is released and the H+ join with NADP+ 8. PSII – ATP, PSI – NADPH 9. ATP (to Calvin cycle), NADPH (to Calvin Cycle), O2 (released ...
Electron transport chain
An electron transport chain (ETC) is a series of compounds that transfer electrons from electron donors to electron acceptors via redox reactions, and couples this electron transfer with the transfer of protons (H+ ions) across a membrane. This creates an electrochemical proton gradient that drives ATP synthesis, or the generation of chemical energy in the form of adenosine triphosphate (ATP). The final acceptor of electrons in the electron transport chain is molecular oxygen.Electron transport chains are used for extracting energy via redox reactions from sunlight in photosynthesis or, such as in the case of the oxidation of sugars, cellular respiration. In eukaryotes, an important electron transport chain is found in the inner mitochondrial membrane where it serves as the site of oxidative phosphorylation through the use of ATP synthase. It is also found in the thylakoid membrane of the chloroplast in photosynthetic eukaryotes. In bacteria, the electron transport chain is located in their cell membrane.In chloroplasts, light drives the conversion of water to oxygen and NADP+ to NADPH with transfer of H+ ions across chloroplast membranes. In mitochondria, it is the conversion of oxygen to water, NADH to NAD+ and succinate to fumarate that are required to generate the proton gradient. Electron transport chains are major sites of premature electron leakage to oxygen, generating superoxide and potentially resulting in increased oxidative stress.