* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Aerobic Respiration - Weber State University
Plant nutrition wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Metalloprotein wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Mitochondrion wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Photosynthesis wikipedia , lookup
Light-dependent reactions wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Electron transport chain wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Biochemistry wikipedia , lookup
Microbial metabolism wikipedia , lookup
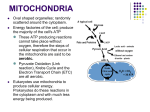
Aerobic Respiration The overall reaction for aerobic respiration is essentially photosynthesis in reverse. Basically, the process involves oxidizing sugar carbons to CO2. Once again, an electron transport system is used to create a hydrogen ion (proton) gradient that is used to make ATP. The final depository for the electrons is O2, which is reduced to water. Aerobic respiration is strongly identified with the mitochondria, however, the starting reactions are in the cytosol. If oxygen is available, the mitochondria get involved with respiration, and the internal structure of the mitochondria is critical to the process. Mitochondria Two membranes: outer membrane and inner membrane Small volume area: intermembrane space Large volume area: matrix The carbon oxidation takes place in two sets of reactions, glycolysis in the cytoplasm and the Krebs cycle in the matrix of the mitochondria. Both glycolysis and the Krebs cycle occur in steps. Stepwise oxidation is important because: 1. dissipate energy that is released as heat 2. generate intermediates ==> steps to start making amino acids, N-bases, other sugars for cell wall and nucleic acids, fatty acids, chlorophyll, anthocyanins, hormones, alkaloids, essential oils, etc. Glycolysis Glycolysis oxidizes glucose or fructose (C6 sugars) to pyruvate (C3 acid). Some of the energy released during the oxidation is captured as ATP. The electrons released by the oxidation are held by NADH. Pyruvate is an organic acid. If the carbon is organic, it is at least partly reduced. Therefore, there are still calories available in pyruvate. The Krebs cycle The Krebs cycle finishes the carbon oxidation process. All of the carbons in pyruvate are oxidized to CO2. It is a cyclic process, with the pyruvate carbons getting attached to an existing cycle molecule prior to oxidation. Once the pyruvate carbons are completely oxidized, the cycle molecule needs to be regenerated. A little bit of ATP is made during the Krebs cycle, but mostly it is an oxidation process. Most of the electrons are held by NADH. Electron Transport Chain (ETC) The electrons from NADH (and other electron holders) are passed to a system of electron carriers in the inner mitochondrial membrane, eventually reaching O2 which is then reduced to H2O. As the electrons pass through the carriers, protons (H+) are moved from the matrix to the intermembrane space. This establishes a proton gradient. When the protons pass through a channel protein back to the matrix, ATP is synthesized. How much ATP gets made? It depends on who is doing the counting. Most plants that oxidize one glucose molecule completely to CO2 will get between 30 and 38 ATP from glycolysis, Krebs cycle, and ETC. The majority of that ATP (85-90%) comes from the ETC. The high end of ATP yield (35-38 ATP) represents about 40% of the energy that was available in a molecule of glucose. The remaining energy is given off as heat. Some plants deliberately have an ATP yield in the mid-teens. Their electrons take an alternative route in the ETC and don’t build as large as proton gradient by the time they get to O2. Because the ATP yield is down, these plants release more heat. These plants are called thermogenic. They can raise the temperature in their microenvironment as much as 10oC. This temperature increase volatilizes scents to attract pollinators more efficiently. Anaerobic respiration Plants are able to respire anaerobically – without oxygen – for a short time. Glycolysis still occurs, but any reactions involving the mitochondria are not available. Without oxygen and the ETC, where can NADH dump off its electrons? Enter fermentation: a set of reactions that let NADH unload electrons so glycolysis can continue. Most plants use alcohol (ethanol) fermentation. The most likely anaerobic scenario for plants ==> roots in water-logged soil. Some plants are specialized to have their roots always in water, like anchored aquatic plants and emerged plants (water lilies, cattails, etc.) The internal anatomy of these plants has large air spaces that form passage ways to get O2 to the roots. Hydrophytes Review Aerobic Respiration Mitochondria structure: outer membrane, inner membrane, intermembrane space, matrix carbon oxidation: glycolysis in the cytosol and the Krebs cycle in the mitochondrial matrix. Be able to briefly state what happens as a result of these two processes. Why is stepwise oxidation of sugar important? What is the importance of generating carbon intermediates during glycolysis and the Krebs cycle? Electron Transport Chain (ETC) Be able to briefly state what happens as a result of this process. thermogenic plants Anaerobic Respiration From a cellular point of view, what is the function of fermentation reactions?