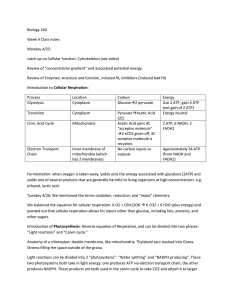
Ch. 9 Cellular Respiration
... Enzyme Complexes Involved in Electron Transport Oxidation/reduction enzymes: 1. NADH dehydrogenase 2. Flavoproteins (FAD) 3. Iron-sulfur proteins 4. Cytochromes 5. Quinones (lipid-soluble) ...
... Enzyme Complexes Involved in Electron Transport Oxidation/reduction enzymes: 1. NADH dehydrogenase 2. Flavoproteins (FAD) 3. Iron-sulfur proteins 4. Cytochromes 5. Quinones (lipid-soluble) ...
Sugárkémiai áttekintés Schiller Róbert
... Chemistry of the hydrated electron - The ideal of the reducing agent: no oxidised product left - the perfect nucleophyilic partner - very selective, in certain cases diffusion controlled rates - previously unknown products, e.g.Ag0, Cu0 ...
... Chemistry of the hydrated electron - The ideal of the reducing agent: no oxidised product left - the perfect nucleophyilic partner - very selective, in certain cases diffusion controlled rates - previously unknown products, e.g.Ag0, Cu0 ...
4 Krebs ETC
... • Cristae contain molecules that make up the electron transport system utilized in aerobic respiration • Electron transport system consists of 4 complexes that receives a pair of electrons then transfers the electrons to the next complex – Cytochrome complex 3 and 4 are membrane bound proteins ...
... • Cristae contain molecules that make up the electron transport system utilized in aerobic respiration • Electron transport system consists of 4 complexes that receives a pair of electrons then transfers the electrons to the next complex – Cytochrome complex 3 and 4 are membrane bound proteins ...
AP Respiration Test Review
... 26. Inside a mitochondrion, trace the pathway of most electrons. 27. What is the primary function of the electron transport chain? 28. What is the direct result of hydrogen ions being pumped from the mitochondrial matrix into the inner membrane space? 29. Where is ATP synthase located? 30. Describe ...
... 26. Inside a mitochondrion, trace the pathway of most electrons. 27. What is the primary function of the electron transport chain? 28. What is the direct result of hydrogen ions being pumped from the mitochondrial matrix into the inner membrane space? 29. Where is ATP synthase located? 30. Describe ...
electron transport chain
... sites between NADH and FP, CoQ or ubiquinone and cytochrome b , cytochrome a a3 &O2 . Therefore 3molecule of ATP are produced from the cycle when started from the beginning (NAD) to the end. While when the electrons enter the respiratory chain from CoQ then 2ATP are formed as it miss or by pass the ...
... sites between NADH and FP, CoQ or ubiquinone and cytochrome b , cytochrome a a3 &O2 . Therefore 3molecule of ATP are produced from the cycle when started from the beginning (NAD) to the end. While when the electrons enter the respiratory chain from CoQ then 2ATP are formed as it miss or by pass the ...
Chapter6summaryHO
... terminal electron acceptor is not available, or because they lack an electron transport chain. E.coli can do anaerobic and aerobic respiration, and fermentation. Streptococcus pneumoniae can only ferment because it lacks an electron transport chain. The only ATP generating pathway is glycolysis and ...
... terminal electron acceptor is not available, or because they lack an electron transport chain. E.coli can do anaerobic and aerobic respiration, and fermentation. Streptococcus pneumoniae can only ferment because it lacks an electron transport chain. The only ATP generating pathway is glycolysis and ...
Respiration Respiration Respiration - Anoka
... transferring a phosphate directly to ADP from another molecule 2. oxidative phosphorylation – use of ATP synthase and energy derived from a proton (H+) gradient to make ATP ...
... transferring a phosphate directly to ADP from another molecule 2. oxidative phosphorylation – use of ATP synthase and energy derived from a proton (H+) gradient to make ATP ...
Carbohydrate Catabolism in the Presence of Oxygen Releases a
... This occurs in a series of redox electron carriers, called the respiratory ...
... This occurs in a series of redox electron carriers, called the respiratory ...
View PDF
... a) Dehydrogenase removes a pair of H atoms (2e and 1p) from sugar (substrate) = oxidation. b) Enzyme in a) delivers the 2e and 1p to coenzyme NAD+. The other p is released as H+ into the solution. NAD+ accepts e’s and is an oxidizing agent. NAD+ is the most useful e acceptor in cellular respiration ...
... a) Dehydrogenase removes a pair of H atoms (2e and 1p) from sugar (substrate) = oxidation. b) Enzyme in a) delivers the 2e and 1p to coenzyme NAD+. The other p is released as H+ into the solution. NAD+ accepts e’s and is an oxidizing agent. NAD+ is the most useful e acceptor in cellular respiration ...
Cytochromes
... ► Electrons carried by reduced NAD and FAD are transported finally to Oxygen (for oxidative phosphorylation) through a gradual downward movement, from high energy level to low energy level. ► A series of Proteins & enzymes Complexes, cytochromes, present in inner membrane of mitochondria, act as tem ...
... ► Electrons carried by reduced NAD and FAD are transported finally to Oxygen (for oxidative phosphorylation) through a gradual downward movement, from high energy level to low energy level. ► A series of Proteins & enzymes Complexes, cytochromes, present in inner membrane of mitochondria, act as tem ...
ch5_SP13x
... • Acidified ( high [H+] ) by action of the Electron Transport Chain (ETC) – H+ are pumped from matrix into this compartment – ATP synthase lets them back into the matrix ...
... • Acidified ( high [H+] ) by action of the Electron Transport Chain (ETC) – H+ are pumped from matrix into this compartment – ATP synthase lets them back into the matrix ...
Key Terms and Ideas: Fill in the blanks or provide a definition in your
... 1. The first law of thermodynamics means ______________; the second law of thermodynamic means ____________________. a. light energy can be harnessed to produce more energy by plants; as disorder increase within a system, more heat is released into the surroundings b. Catabolic and anabolic reaction ...
... 1. The first law of thermodynamics means ______________; the second law of thermodynamic means ____________________. a. light energy can be harnessed to produce more energy by plants; as disorder increase within a system, more heat is released into the surroundings b. Catabolic and anabolic reaction ...
View PDF
... Chemiosmosis: The Energy-Coupling Mechanism • Electron transfer in the electron transport chain causes proteins to pump H+ from the mitochondrial matrix to the intermembrane space. • H+ then moves back across the membrane, passing through the protein, ATP synthase. • ATP synthase uses the ex ...
... Chemiosmosis: The Energy-Coupling Mechanism • Electron transfer in the electron transport chain causes proteins to pump H+ from the mitochondrial matrix to the intermembrane space. • H+ then moves back across the membrane, passing through the protein, ATP synthase. • ATP synthase uses the ex ...
Week 4:
... Fermentation: when oxygen is taken away, yields only the energy associated with glycolysis (2ATP) and yields one of several products that are generally harmful to living organisms at high concentrations: e.g. ethanol, lactic acid. Tuesday 4/26: We mentioned the terms oxidation, reduction, and “redox ...
... Fermentation: when oxygen is taken away, yields only the energy associated with glycolysis (2ATP) and yields one of several products that are generally harmful to living organisms at high concentrations: e.g. ethanol, lactic acid. Tuesday 4/26: We mentioned the terms oxidation, reduction, and “redox ...
Freeman 1e: How we got there
... • Chemolithotrophs use inorganic compounds as electron donors, whereas phototrophs use light to form a proton motive force. The proton motive force is involved in all forms of respiration and photosynthesis (Figure 5.23). ...
... • Chemolithotrophs use inorganic compounds as electron donors, whereas phototrophs use light to form a proton motive force. The proton motive force is involved in all forms of respiration and photosynthesis (Figure 5.23). ...
second exam 05
... phosphorylation. This process can be best characterized by which statement? a) ATP is generated by electron transfer from quinones to ADP which then reacts with a phosphate molecule b) The membrane potential generated during electron transport in the mitochondria increases the chemical potential of ...
... phosphorylation. This process can be best characterized by which statement? a) ATP is generated by electron transfer from quinones to ADP which then reacts with a phosphate molecule b) The membrane potential generated during electron transport in the mitochondria increases the chemical potential of ...
L3 - Bacterial Metabolism v3
... • The incomplete breakdown of glucose with an organic compound serving as the final electron acceptor • Only pathway operating is glycolysis ...
... • The incomplete breakdown of glucose with an organic compound serving as the final electron acceptor • Only pathway operating is glycolysis ...
Chapter 17
... 23. “Uncouple” agents, 2,4-dinitrophenol (DNP) and carbonylcyanide-ptrifluoromethoxyphenylhydrazone (FCCP), bear H+ and diffuse into the matrix. Thus, these uncouple agents reduce the electrochemical potential across the inner mitochondrial membrane, and consequently inhibit ATP synthesis. 24. Uncou ...
... 23. “Uncouple” agents, 2,4-dinitrophenol (DNP) and carbonylcyanide-ptrifluoromethoxyphenylhydrazone (FCCP), bear H+ and diffuse into the matrix. Thus, these uncouple agents reduce the electrochemical potential across the inner mitochondrial membrane, and consequently inhibit ATP synthesis. 24. Uncou ...
Oxidative phosphorylation (1)
... named the electron transport chain, ECT (Respiratory chain). • As electrons are passed down the electron transport chain, they lose ...
... named the electron transport chain, ECT (Respiratory chain). • As electrons are passed down the electron transport chain, they lose ...
Cellular respiration includes three pathways
... 28. In the next redox reaction flavoprotein returns to its _______________form as it passes electrons(loses electrons) to an iron-sulfur protein. 29. ______________________ (Q), is a small hydophobic molecule that is mobile within the membrane. 30. _________________________adds electrons in Complex ...
... 28. In the next redox reaction flavoprotein returns to its _______________form as it passes electrons(loses electrons) to an iron-sulfur protein. 29. ______________________ (Q), is a small hydophobic molecule that is mobile within the membrane. 30. _________________________adds electrons in Complex ...
MEMBRANE-BOUND ELECTRON TRANSFER AND ATP …
... Electrons are carried from Complex III to Complex IV by cytochrome c, a small hydrophilic peripheral membrane protein located on the cytosolic or P side of the IMM. Complex II (Succinate-UQ oxidoreductase) is membrane bound and contains the FADH2 as a prosthetic group . So electrons from FADH2 feed ...
... Electrons are carried from Complex III to Complex IV by cytochrome c, a small hydrophilic peripheral membrane protein located on the cytosolic or P side of the IMM. Complex II (Succinate-UQ oxidoreductase) is membrane bound and contains the FADH2 as a prosthetic group . So electrons from FADH2 feed ...
Electron transport chain
An electron transport chain (ETC) is a series of compounds that transfer electrons from electron donors to electron acceptors via redox reactions, and couples this electron transfer with the transfer of protons (H+ ions) across a membrane. This creates an electrochemical proton gradient that drives ATP synthesis, or the generation of chemical energy in the form of adenosine triphosphate (ATP). The final acceptor of electrons in the electron transport chain is molecular oxygen.Electron transport chains are used for extracting energy via redox reactions from sunlight in photosynthesis or, such as in the case of the oxidation of sugars, cellular respiration. In eukaryotes, an important electron transport chain is found in the inner mitochondrial membrane where it serves as the site of oxidative phosphorylation through the use of ATP synthase. It is also found in the thylakoid membrane of the chloroplast in photosynthetic eukaryotes. In bacteria, the electron transport chain is located in their cell membrane.In chloroplasts, light drives the conversion of water to oxygen and NADP+ to NADPH with transfer of H+ ions across chloroplast membranes. In mitochondria, it is the conversion of oxygen to water, NADH to NAD+ and succinate to fumarate that are required to generate the proton gradient. Electron transport chains are major sites of premature electron leakage to oxygen, generating superoxide and potentially resulting in increased oxidative stress.