Lesson element
... molecule C6H12O6 into two pyruvate molecules. The formula for pyruvate is C3H4O3. It does not require oxygen and does not release carbon dioxide; it does produce sufficient energy to make two ATP molecules per glucose molecule. Any chemical compound which can be broken down during respiration to rel ...
... molecule C6H12O6 into two pyruvate molecules. The formula for pyruvate is C3H4O3. It does not require oxygen and does not release carbon dioxide; it does produce sufficient energy to make two ATP molecules per glucose molecule. Any chemical compound which can be broken down during respiration to rel ...
Student Module_4
... Fuel Utilization During Exercise • The chemical reactions that use these substances to make energy are called metabolism. • Two interrelated energy-producing systems: – Aerobic: requiring oxygen. – Anaerobic: not requiring oxygen • Inefficient; generates lactic acid that can be converted into an en ...
... Fuel Utilization During Exercise • The chemical reactions that use these substances to make energy are called metabolism. • Two interrelated energy-producing systems: – Aerobic: requiring oxygen. – Anaerobic: not requiring oxygen • Inefficient; generates lactic acid that can be converted into an en ...
Macromolecule WebQuest
... answer the questions below as they will help you understand the different structures and functions of biological molecules. Follow the link ...
... answer the questions below as they will help you understand the different structures and functions of biological molecules. Follow the link ...
The Citric Acid Cycle
... • Step 2 Citrate is isomerized into isocitrate (get the six-carbon unit ready for oxidative decarboxylation) via a dehydration step followed by a hydration step; cis-aconitate (顺乌头酸) is an intermediate during this transformation, thus the catalytic enzyme is named as aconitase, which contains a 4Fe ...
... • Step 2 Citrate is isomerized into isocitrate (get the six-carbon unit ready for oxidative decarboxylation) via a dehydration step followed by a hydration step; cis-aconitate (顺乌头酸) is an intermediate during this transformation, thus the catalytic enzyme is named as aconitase, which contains a 4Fe ...
Fatty Acid & Protein Metabolism
... • Lack of insulin causes hyperglycemia • Dehydration and sweet taste to urine ...
... • Lack of insulin causes hyperglycemia • Dehydration and sweet taste to urine ...
3.2 Metabolism of cardiac muscle cell
... Myocardium is able to produce energy from several substrates: fatty acids, glucose, lactate, pyruvate, ketone bodies and even aminoacids. Preference of individual substrates representing the particular sources of energy depends on their current concentration in both blood and cardiac muscle cells. T ...
... Myocardium is able to produce energy from several substrates: fatty acids, glucose, lactate, pyruvate, ketone bodies and even aminoacids. Preference of individual substrates representing the particular sources of energy depends on their current concentration in both blood and cardiac muscle cells. T ...
5_Bio_1_ReKaps
... i) Sex-linked recessive due to more males affected, generation skipping ii) Females can be carriers and should be tested. Males do not need to be tested because they cannot be carriers; would either display trait or have no disease alleles. iii) Gen4 male has 25% chance of being affected. (There is ...
... i) Sex-linked recessive due to more males affected, generation skipping ii) Females can be carriers and should be tested. Males do not need to be tested because they cannot be carriers; would either display trait or have no disease alleles. iii) Gen4 male has 25% chance of being affected. (There is ...
Finals Practice Exam answers
... VI). Design a molecule that resembles the proposed transition state and check to see if it inhibits or binds to the enzyme stronger than the substrate or product (transition state analog). Many of the best drugs are transition state analogs. ...
... VI). Design a molecule that resembles the proposed transition state and check to see if it inhibits or binds to the enzyme stronger than the substrate or product (transition state analog). Many of the best drugs are transition state analogs. ...
Biochemistry - El Camino College
... 1. ___________ - storage form of glucose in animals; stored in our ______ and muscle cells, broken down to glucose when needed 2. __________ - storage form of glucose in plants; stored in starch granules in plant cells, digested to __________ in our bodies 3. ___________ - main component of plant ce ...
... 1. ___________ - storage form of glucose in animals; stored in our ______ and muscle cells, broken down to glucose when needed 2. __________ - storage form of glucose in plants; stored in starch granules in plant cells, digested to __________ in our bodies 3. ___________ - main component of plant ce ...
PASS MOCK EXAM
... 28. How many molecules of NAD+ are reduced to NADH when the citric acid cycle processes the products of a single molecule of glucose? A) 3 B) 6 C) 9 D) 2 E) 4 ...
... 28. How many molecules of NAD+ are reduced to NADH when the citric acid cycle processes the products of a single molecule of glucose? A) 3 B) 6 C) 9 D) 2 E) 4 ...
nerves & action potentials - IB
... • Other blood vessels receive blood after liver hepatocytes action • 2 hormones • Insulin antagonistic • glucagon ...
... • Other blood vessels receive blood after liver hepatocytes action • 2 hormones • Insulin antagonistic • glucagon ...
Biological_Molecules worksheet - answers
... a. They make up cell membranes b. Long term energy source – they release as twice as much energy as carbohydrates/protein. c. Good thermal insulators, reducing heat loss. ...
... a. They make up cell membranes b. Long term energy source – they release as twice as much energy as carbohydrates/protein. c. Good thermal insulators, reducing heat loss. ...
Biology – Unit 3 Review
... energy and use it to ultimately create food that can be used by their own organism or other organisms that may consume them. Light energy, carbon dioxide and water are needed for the organism to produce glucose and ATP. Oxygen is given off as a waste product. The entire process is divided into two d ...
... energy and use it to ultimately create food that can be used by their own organism or other organisms that may consume them. Light energy, carbon dioxide and water are needed for the organism to produce glucose and ATP. Oxygen is given off as a waste product. The entire process is divided into two d ...
topic 2 powerpoint
... • Cells make a copy of their DNA during the S phase of their cell cycle. • Molecules needed for the process include enzymes and free nucleotides. • The first step of replication involves the separation of the double helix into two strands using the enzyme helicase. • Helicase separates the strands b ...
... • Cells make a copy of their DNA during the S phase of their cell cycle. • Molecules needed for the process include enzymes and free nucleotides. • The first step of replication involves the separation of the double helix into two strands using the enzyme helicase. • Helicase separates the strands b ...
BIO121_Chapter 6
... B. Fermentation generates more ATP per glucose than aerobic cellular respiration. C. Fermentation does not generate toxic byproducts such as CO2. D. Fermentation gets rid of pyruvate, which would otherwise accumulate in the cell. ...
... B. Fermentation generates more ATP per glucose than aerobic cellular respiration. C. Fermentation does not generate toxic byproducts such as CO2. D. Fermentation gets rid of pyruvate, which would otherwise accumulate in the cell. ...
131110 COS ATP - Community of Reason
... Energy is defined as the capacity to do work, i.e. to move matter. Cells, tissues and organisms need energy for a variety of processes ...
... Energy is defined as the capacity to do work, i.e. to move matter. Cells, tissues and organisms need energy for a variety of processes ...
File
... (D) A beta-linked disaccharide Answer = A 2. Cell communication is critical for the function of both unicellular and multicellular eukaryotes. Which of the following is likely true of cell signaling? A. cell signaling uses the highest molecular weight molecules found in living cells B. Cell signalin ...
... (D) A beta-linked disaccharide Answer = A 2. Cell communication is critical for the function of both unicellular and multicellular eukaryotes. Which of the following is likely true of cell signaling? A. cell signaling uses the highest molecular weight molecules found in living cells B. Cell signalin ...
electron transport chain
... -are oxidations – loss of electrons -are also dehydrogenations lost electrons are accompanied by hydrogen what is actually lost is a hydrogen atom (1 electron, 1 proton). • A hydrogen atom is equivalent to a hydrogen ion plus an electron H = H+ + e-electrons carry energy from one molecule to another ...
... -are oxidations – loss of electrons -are also dehydrogenations lost electrons are accompanied by hydrogen what is actually lost is a hydrogen atom (1 electron, 1 proton). • A hydrogen atom is equivalent to a hydrogen ion plus an electron H = H+ + e-electrons carry energy from one molecule to another ...
Biochemistry 2 [1203253] intended learning outcomes DNA, RNA
... the Pentose Phosphate Pathway Generates NADPH and Synthesizes FiveCarbon Sugars The Metabolism of Glucose 6-Phosphate by the Pentose Phosphate Pathway Is Coordinated with Glycolysis Glucose 6-Phosphate Dehydrogenase Plays a Key Role in Protection Against Reactive Oxygen Species Glycogen, hexos ...
... the Pentose Phosphate Pathway Generates NADPH and Synthesizes FiveCarbon Sugars The Metabolism of Glucose 6-Phosphate by the Pentose Phosphate Pathway Is Coordinated with Glycolysis Glucose 6-Phosphate Dehydrogenase Plays a Key Role in Protection Against Reactive Oxygen Species Glycogen, hexos ...
1. What is the collective term for all of the chemical processes
... 42. Which of the following is the proper order of DNA Replication/Protein Synthesis A) Transcription, Translation, Proteins to form new DNA from existing DNA B) Protein placement, Transcription, Translation C) Translation, Transcription, DNA polymerase formation D) Proteins to form new DNA from exis ...
... 42. Which of the following is the proper order of DNA Replication/Protein Synthesis A) Transcription, Translation, Proteins to form new DNA from existing DNA B) Protein placement, Transcription, Translation C) Translation, Transcription, DNA polymerase formation D) Proteins to form new DNA from exis ...
sample
... act as a source of electrons and protons. Figure 9 shows the electron carriers at progressively lower energy levels. As electrons pass along the chain of carriers in a series of redox reactions, they release energy. This energy is used to synthesise ATP by oxidative phosphorylation. Oxygen is the te ...
... act as a source of electrons and protons. Figure 9 shows the electron carriers at progressively lower energy levels. As electrons pass along the chain of carriers in a series of redox reactions, they release energy. This energy is used to synthesise ATP by oxidative phosphorylation. Oxygen is the te ...
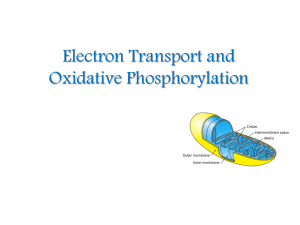
Electron Transport and Oxidative Phosphorylation
... The NADH and FADH2 formed in glycolysis, fatty acid oxidation, and the citric acid cycle are energy-rich molecules. because each contains a pair of electrons having a high transfer potential. ...
... The NADH and FADH2 formed in glycolysis, fatty acid oxidation, and the citric acid cycle are energy-rich molecules. because each contains a pair of electrons having a high transfer potential. ...
Metabolism
... Covalent bonds (in which two atoms share electrons) are the strongest bonds within protein and exist in the primary structure itself. Covalent bonds can also exist as disulphide bridges. These occur when cysteine side chains within a protein are oxidised resulting in a covalent link between the two ...
... Covalent bonds (in which two atoms share electrons) are the strongest bonds within protein and exist in the primary structure itself. Covalent bonds can also exist as disulphide bridges. These occur when cysteine side chains within a protein are oxidised resulting in a covalent link between the two ...
Glycolysis
Glycolysis (from glycose, an older term for glucose + -lysis degradation) is the metabolic pathway that converts glucose C6H12O6, into pyruvate, CH3COCOO− + H+. The free energy released in this process is used to form the high-energy compounds ATP (adenosine triphosphate) and NADH (reduced nicotinamide adenine dinucleotide).Glycolysis is a determined sequence of ten enzyme-catalyzed reactions. The intermediates provide entry points to glycolysis. For example, most monosaccharides, such as fructose and galactose, can be converted to one of these intermediates. The intermediates may also be directly useful. For example, the intermediate dihydroxyacetone phosphate (DHAP) is a source of the glycerol that combines with fatty acids to form fat.Glycolysis is an oxygen independent metabolic pathway, meaning that it does not use molecular oxygen (i.e. atmospheric oxygen) for any of its reactions. However the products of glycolysis (pyruvate and NADH + H+) are sometimes disposed of using atmospheric oxygen. When molecular oxygen is used in the disposal of the products of glycolysis the process is usually referred to as aerobic, whereas if the disposal uses no oxygen the process is said to be anaerobic. Thus, glycolysis occurs, with variations, in nearly all organisms, both aerobic and anaerobic. The wide occurrence of glycolysis indicates that it is one of the most ancient metabolic pathways. Indeed, the reactions that constitute glycolysis and its parallel pathway, the pentose phosphate pathway, occur metal-catalyzed under the oxygen-free conditions of the Archean oceans, also in the absence of enzymes. Glycolysis could thus have originated from chemical constraints of the prebiotic world.Glycolysis occurs in most organisms in the cytosol of the cell. The most common type of glycolysis is the Embden–Meyerhof–Parnas (EMP pathway), which was discovered by Gustav Embden, Otto Meyerhof, and Jakub Karol Parnas. Glycolysis also refers to other pathways, such as the Entner–Doudoroff pathway and various heterofermentative and homofermentative pathways. However, the discussion here will be limited to the Embden–Meyerhof–Parnas pathway.The entire glycolysis pathway can be separated into two phases: The Preparatory Phase – in which ATP is consumed and is hence also known as the investment phase The Pay Off Phase – in which ATP is produced.↑ ↑ 2.0 2.1 ↑ ↑ ↑ ↑ ↑ ↑


















![Biochemistry 2 [1203253] intended learning outcomes DNA, RNA](http://s1.studyres.com/store/data/002558734_1-17434e4debf95f3be87a42da9306bb2f-300x300.png)